List of Benzodiazepines: DBZDs & Rx Medications
An unbiased, evidence-based resource for the purpose of harm-reduction & responsible benzodiazepine consumption.
Benzodiazepines (AKA “BZDs” or “Benzos”) are a large class of psychoactive drugs used for the treatment of anxiety and insomnia.
Here, we highlight the effects of benzos for both medical and recreational purposes.
Warning: The health risks of benzodiazepines shouldn’t be taken lightly. These compounds are particularly problematic. Dependence forms quickly and can have a profoundly negative impact on the user’s mental and physical wellbeing.
Mixing benzos with alcohol or other GABAergic or opiate drugs can be fatal.
What Are Benzodiazepines?
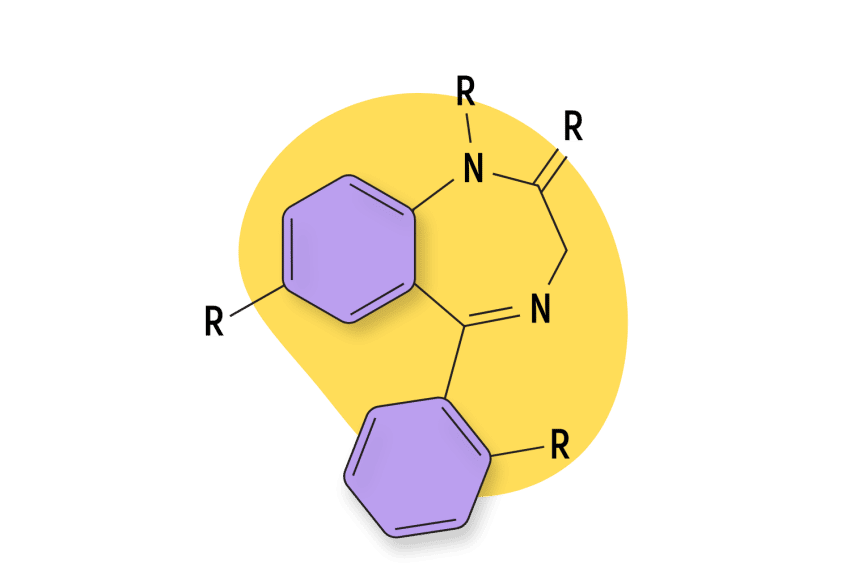
A benzodiazepine refers to any chemical that includes a benzene ring fused to a diazepine ring [128].
Most of the drugs in this class work by slowing down brain activity. This gives them hypnotic, anxiolytic, muscle-relaxant, and in some cases, intoxicating qualities. The most common benzos today are Xanax, Valium, Librium, and Klonopin. There is also a large selection of designer benzodiazepines (called DBZDs for short) — which refer to benzodiazepine compounds that have not been formally tested.
Most benzodiazepine drugs work by enhancing the effects of the neurotransmitter GABA on the GABA-A receptors.
GABA is the primary neuro-inhibitory molecule in the brain — meaning it works to reduce brain activity. When brain activity is reduced, we feel less anxiety and more sedated. Benzos act as a “chill pill” — removing the constant sense of worry and allowing users to temporarily stop caring about their problems.
This fades when the drug’s effect wears off.
Unfortunately, inhibiting brain activity affects other areas of cognition as well:
- Benzos inhibit memory formation — causing frequent lapses in memory or “blackouts.”
- Benzos block one’s ability to form complex or abstract thoughts.
- Benzos induce a state of disinhibition similar to alcohol — causing people may say or do things they normally wouldn’t.
Some of the more potent benzodiazepines are used in surgery — such as midazolam and remimazolam. These drugs inhibit the ability to form memories and act as sedatives.
List of Common Benzodiazepines:
| Image | Compound Name | Status | Half-Life | Equivalent Dose to 10 mg Diazepam |
|---|---|---|---|---|
| Alprazolam (Xanax) | Approved 💊 | Short-Acting (11-13 hours) | 0.75 mg | |
| Bromazepam (Lectopam) | Approved 💊 | Intermediate-Acting (12–20 hours) | 5 mg | |
| Brotizolam (Lendormin) | Approved 💊 | Short-Acting (4–5 hours) | 0.2 mg | |
| Chlordiazepoxide (Librium) | Approved 💊 | Long-Acting (24–36 hours) | 25 mg | |
| Clobazam (Onfi) | Approved 💊 | Intermediate-Acting (6–12 hours) | 20 mg | |
| Clonazepam (Klonopin) | Approved 💊 | Intermediate-Acting (9.5–50 hours) | 0.75 mg | |
| Clorazepate (Tranxene) | Approved 💊 | Long-Acting (32–152 hours) | 13 mg | |
| Desmethylflunitrazepam | RC 🧪 | Unknown | 10 mg | |
| Diazepam (Valium) | Approved 💊 | Long-Acting (24–36 hours) | 10 mg | |
| Diclazepam | RC 🧪 | Intermediate-Acting (42 hours) | 1 mg | |
| Estazolam (ProSom) | Approved 💊 | Intermediate-Acting (7–12 hours) | 1 mg | |
| Etizolam (Etilaam) | Approved 💊 | Short-Acting (5-7 hours) | 1 mg | |
| Flubromazepam | RC 🧪 | Long-Acting (100–220 hours) | 6 mg | |
| Flubromazolam (JYI-73) | RC 🧪 | Intermediate-Acting (6–12 hours) | 0.2 mg | |
| Flunitrazepam (Rohypnol) | Approved 💊 | Long-Acting (15–35 hours) | 1 mg | |
| Flunitrazolam | RC 🧪 | Short-Acting (5-13 hours) | 0.1 mg | |
| Flurazepam (Dalmadorm) | Approved 💊 | Long-Acting (47–100 hours) | 20 mg | |
| Flutoprazepam (Restas) | Approved 💊 | Long-Acting (60-90 hours) | 2.5 mg | |
| Halazepam (Paxipam) | Approved 💊 | Intermediate-Acting (8–14 hours) | 20 mg | |
| Ketazolam (Anseren) | Approved 💊 | Long-Acting (30-100 hours) | 15 mg | |
| Loprazolam (Dormonoct) | Approved 💊 | Short-Acting (6–20 hours) | 1 mg | |
| Lorazepam (Ativan) | Approved 💊 | Intermediate-Acting (12–18 hours) | 1.3 mg | |
| Lormetazepam (Noctamid) | Approved 💊 | Short-Acting (10-12 hours) | 1 mg | |
| Medazepam (Azepamid) | Approved 💊 | Long-Acting (36–200 hours) | 10 mg | |
| Metaclazepam (Talis) | Approved 💊 | Intermediate-Acting (6–12 hours) | 10 mg | |
| Midazolam (Versed) | Approved 💊 | Short-Acting (2-6 hours) | 10 mg | |
| Nitrazepam (Mogadon) | Approved 💊 | Intermediate-Acting (24–40 hours) | 10 mg | |
| Nordiazepam (Madar) | Approved 💊 | Long-Acting (30–150 hours) | 10 mg | |
| Oxazepam (Serax) | Approved 💊 | Intermediate-Acting (10–20 hours) | 20 mg | |
| Phenazepam | RC 🧪 | Long-Acting (60 hours) | 1 mg | |
| Prazepam (Centrax) | Approved 💊 | Long-Acting (36–200 hours) | 15 mg | |
| Pyrazolam | RC 🧪 | Intermediate-Acting (16–18 hours) | 1 mg | |
| Quazepam (Doral) | Approved 💊 | Long-Acting (39–120 hours) | 25 mg | |
| Temazepam (Restoril) | Approved 💊 | Intermediate-Acting (10–15 hours) | 20 mg | |
| Triazolam (Halcion) | Approved 💊 | Short-Acting (2–3 hours) | 0.3 mg |
Guidelines For the Responsible Use of Benzodiazepines
- 🥣 Don’t mix — Mixing benzodiazepines with other depressants (alcohol, GHB, phenibut, barbiturates, opiates) can be fatal.
- ⏳ Take frequent breaks or plan for a short treatment span — Benzodiazepines can form dependence quickly, so it’s important to stop using the drug periodically.
- 🥄 Always stick the proper dose — The dosage of benzos can vary substantially. Some drugs require 20 or 30 mg, others can be fatal in doses as low as 3 mg.
- 💊 Be aware of contraindications — Benzodiazepines are significantly more dangerous in older people or those with certain medical conditions.
- 🧪 Test your drugs — If ordering benzos from unregistered vendors (online or street vendors), order a benzo test kit to ensure your pills contain what you think they do.
- 💉 Never snort or inject benzos — Not only does this provide no advantage, but it’s also extremely dangerous. Benzos should be taken orally.
- 🌧 Recognize the signs of addiction — Early warning signs are feeling like you’re not “yourself” without the drug or hiding your habits from loved ones.
- ⚖️ Understand the laws where you live — In most parts of the world, benzodiazepines are only considered legal if given a prescription by a medical doctor.
- 📞 Know where to go if you need help — Help is available for benzodiazepine addiction; you just have to ask for it. Lookup “addiction hotline” for more information where you live. (USA: 1-800-662-4357; Canada: 1-866-585-0445; UK: 0300-999-1212).
What Are the Different Types of Benzodiazepines?
The benzodiazepine class contains many drugs with diverse effect profiles.
Most benzos are anxiolytic, sedative, and muscle-relaxant, but there are also members in this family that have antibiotic, antifungal, analgesic, nootropic, and anti-inflammatory qualities.
There are a few different ways to categorize the different types of benzos.
In terms of chemical structure, there are 14 different types of benzos:
- 1,4-Benzodiazepines
- 1,5-Benzodiazepines
- 2,3-Benzodiazepines
- Triazolobenzodiazepines
- Imidazobenzodiazepines
- Oxazolobenzodiazepines
- Thienodiazepines
- Thienobenzodiazepines
- Pyridodiazepines
- Pyrazolodiazepines
- Pyrrolodiazepines
- Pyrrolobenzodiazepines
- Tetrahydroisoquinobenzodiazepines
- Benzodiazepine Prodrugs
The most common method of grouping benzodiazepines is by their duration of effects (short, intermediate, or long-acting). This is because the duration of effects is the best factor for determining what the drug should be used for and what level of risk for addiction or overdose it carries.
The “action” of a benzodiazepine is determined by its half-life (how long it takes for half the drug to be eliminated from the body).
Benzos can be classified into three main groups depending on the duration of effects.
1. Short-Acting Benzodiazepines
Short-acting benzodiazepines enter and leave the body quickly.
Anything with a half-life between 1 and 12 hours is classified under this group. This means the drug remains in the system for around 24 hours or less.
Short-acting benzos are usually preferred for treating insomnia because they tend to kick in quickly to help the patient get to sleep but wear off long before the patient wakes up. Most benzodiazepines interfere with REM sleep, so the sooner it wears off while asleep, the better.
These drugs have the lowest risk for accumulation and overdose with repeated use but are at high risk for abuse and addiction because users need to redose more often. Many of the drugs in this class have been replaced with a newer class of hypnotic drugs called Z-drugs, which have less of an impact on deep sleep [44].
Short-acting benzodiazepines:
| Drug Name | Half-Life | Estimated Duration of Effects |
| Alprazolam (Xanax) | 11-13 hours | 5–8 hours |
| Brotizolam (Lendormin) | 4–5 hours | 4-8 hours |
| Camazepam | 6–11 hours | 8–12 hours |
| Clotiazepam | 4–10 hours | 4–8 hours |
| Doxefazepam | 3-4 hours | 2–8 hours |
| Flunitrazolam | 5-13 hours | 4-8 hours |
| Loprazolam (Dormonoct) | 6–20 hours | 6–10 hours |
| Lormetazepam (Loramet) | 10-12 hours | 4-8 hours |
| Midazolam (Versed) | 2-6 hours | 4-8 hours |
| Oxazolam | 6–12 hours | 6–12 hours |
| Triazolam (Halcion) | 2–3 hours | 4-7 hours |
2. Intermediate-Acting Benzodiazepines
Intermediate-acting benzodiazepines have a median half-life between 12 and 40 hours. This means these drugs can remain in the system anywhere from 1 to 3 days.
This group is also popular for managing anxiety and insomnia and are often prescribed instead of short-acting benzos as a way to lower the risk of the patient forming dependence on the drug.
Intermediate-acting benzodiazepines:
| Drug Name | Half-Life | Estimated Duration of Effects |
| Bromazepam (Lectopam) | 20–40 hours | 12–20 hours |
| Cinolazepam | 8–12 hours | 8–12 hours |
| Clobazam (Onfi) | 8–60 hours | 6–12 hours |
| Estazolam (ProSom) | 10–24 hours | 7–12 hours |
| Flubromazolam (JYI-73) | 10-20 hours | 6–12 hours |
| Flunitrazepam (Rohypnol) | 15–35 hours | 8–12 hours |
| Lorazepam (Ativan) | 12–18 hours | 11–20 hours |
| Metaclazepam (Talis) | 4–5 hours | 6–12 hours |
| Oxazepam (Serax) | 10–20 hours | 6–9 hours |
| Temazepam (Restoril) | 10–15 hours | 6–10 hours |
| Tetrazepam | 8–24 hours | 6–10 hours |
3. Long-Acting Benzodiazepines
Long-acting benzodiazepines are often used for things like anesthesia during surgery, managing alcohol withdrawal syndrome, or long-term anxiety support.
These drugs take a long time to leave the body and tend to leave patients with a hangover effect when taking them to manage anxiety or insomnia.
In order to classify as a long-acting benzodiazepine, the drug must have a half-life between 40 and 125 hours. These drugs can remain in the system for several days.
Long-acting benzodiazepines:
| Drug Name | Half-Life | Estimated Duration of Effects |
| Chlordiazepoxide (Librium) | 24–48 hours | 24–36 hours |
| Clorazepate (Tranxene) | 32–152 hours | 8–12 hours |
| Diazepam (Valium) | 32–205 hours | 24–36 hours |
| Flurazepam (Dalmadorm) | 47–100 hours | 12–16 hours |
| Halazepam (Paxipam) | 15-35 hours | 30–100 hours |
| Mexazolam | 48–72 hours | 6-12 hours |
| Nitrazepam (Mogadon) | 24–40 hours | 6–8 hours |
| Nordiazepam (Madar) | 30–150 hours | 10–20 hours |
| Phenazepam (Phenazepam) | 60 hours | 18+ hours |
| Prazepam (Centrax) | 36–200 hours | 8–20 hours |
| Quazepam (Doral) | 39–120 hours | 6–12 hours |
Are Benzodiazepines Safe? What Are The Risks?
Benzodiazepines were believed to be a miracle drug in the 1970s. In 1977 they were dubbed the most prescribed medication in America. Today, we know this isn’t the case — benzodiazepines have a place in medicine and can certainly be used safely and responsibly — but they also carry a significant risk for addiction and abuse.
Compulsive benzo consumption can lead to problems with learning and memory, as well as lowered immunity and certain types of cancers. It’s also common for people to experience “blackouts” — during which they do or say things that land them in legal trouble or cause conflict with friends and family.
Overdoses on benzos alone are rare, but the risk increases significantly when mixing with other drugs or alcohol.
Here’s what makes benzodiazepines so dangerous.
1. Risk of Overdose
When used at the right dose and for short periods of time (up to 4 weeks), benzodiazepines are regarded as safe [1]. It’s exceedingly rare for someone to die from a benzodiazepine overdose if it was the only substance used. However, the risk of death increases substantially if alcohol, phenibut, GHB, opiates, Z-drugs, or other sedatives are used in combination. Roughly 75% of all benzodiazepine-related deaths involve a combination of benzodiazepines with opiate drugs [7].
Despite being less “directly” deadly, benzodiazepine overdoses will still result in widespread neurological damage with lasting consequences on memory, mood, and cognition [6].
The signs of benzodiazepine overdose include:
- Drowsiness
- Slurred speech
- Nystagmus
- Hypotension
- Ataxia
- Coma
- Respiratory depression
- Cardiorespiratory arrest
There is an antidote for benzos used in emergency medicine called flumazenil (Anexate), but it doesn’t always work. The antidote becomes less effective in people who use benzos frequently — unfortunately, these are also the users who are most likely to need it.
2. Potential For Addiction & Abuse
Tolerance and dependence on benzodiazepine can happen surprisingly quickly.
The body adapts to the effects of benzodiazepines after just a few weeks. As the body adapts, it becomes dependent, leading to withdrawal symptoms as the drugs wear off. This is why benzodiazepines are normally only prescribed for one or two weeks at a time.
As dependence develops, it becomes increasingly difficult to stop taking the drug.
Benzodiazepine addiction is significantly more common with misuse, and misuse is common. According to the National Institute on Drug Abuse (NIDA), nearly 1 in 5 benzodiazepine users misuse the drug.
The trick to using these drugs safely is to take them only when necessary and to take breaks often to prevent dependency from forming.
3. Long-Term Consequences of Benzodiazepines
The most substantial consequences of benzodiazepines come from repeated, long-term use. Problems with memory, concentration, and the development of paradoxical symptoms like anxiety and insomnia are the most common.
There are also a host of other health conditions associated with long-term benzodiazepine use:
- Benzodiazepines increase the risk of suicide [2]
- Benzodiazepines are associated with early-onset dementia [3]
- Benzodiazepine use increases the risk of respiratory tract infection [4]
- Benzodiazepine increase the risk of certain types of cancer [5]
Long-term side effects of benzodiazepines include:
- Agoraphobia
- Anterograde amnesia (difficulty forming new memories)
- Cognitive impairment
- Decreased IQ
- Depression
- Inability to think creatively
- Loss of sex-drive
- Social anxiety
4. Effects of Disinhibition
It’s common for people who abuse benzodiazepines to do or say things they regret while under the influence of the drug. Users often embarrass themselves, hurt others (verbally or physically), or get into serious trouble (legally or otherwise).
This is caused by a concept called disinhibition. This effect is common with GABAergic drugs, including alcohol and GHB. It leads to a loss of control over socially unacceptable behavior. Users essentially lose the little voice in their head, preventing them from doing whatever the unconscious mind desires. Their ability to read social cues empathize with others, or control outbursts is also temporarily lost.
Often, users who say or do things they normally don’t have any memory of the event as the drugs also block one’s capacity to form memories while under their effects.
5. Contraindications (When To Avoid Benzodiazepines For Any Reason)
Certain medical conditions rule out the ability to use benzos safely. Other drugs, such as Z-drugs or other sedatives, are used instead for these cases.
Contraindications for benzodiazepines include:
- Myasthenia gravis
- Sleep apnea
- Bronchitis
- Chronic obstructive pulmonary disease (COPD)
- Personality disorders
- Intellectual disabilities due to frequent paradoxical reactions
- Major depression
- Conjunctive use of barbiturates, opiates, or those suffering from alcoholism
- Over the age of 65 (high risk)
Are Benzodiazepines Legal?
In the US, benzodiazepines are classified as a Schedule IV drug. This implies a high risk for abuse but clear medical benefit. You can only get drugs in this class by getting a prescription from a doctor. All benzos are considered Schedule IV, even those that have not been approved by the FDA (including research chemicals like Bromazepam and Clonazolam).
In Canada, the possession of personal quantities of benzodiazepines is considered legal. These compounds are classified as Schedule IV medicines.
In the UK, benzos are classified as a Class C drug. The illegal distribution of benzos can lead to a 14-year prison sentence and unlawful possession up to 7 years.
Most countries around the world classify benzos as Schedule IV or equivalent. This class is reserved for drugs that carry the risk of abuse and addiction but have a well-defined place in medicine.
What Are Designer Benzodiazepines (DBZD)?
The term “designer drug” or “research chemical” refers to any substance that was created in the likeness of a restricted substance for the purpose of bypassing regulations. Designer drugs are usually active analogs of known controlled substances. They’re made by modifying existing benzos, such as estizolam or alprazolam, while others are made by creating prodrugs of existing benzos.
Note: a prodrug refers to an inactive compound that’s converted to the active form of the drug after it’s processed by the liver.
In the world of benzodiazepines, most of the designer drugs entering the market today are simply re-discovered compounds developed in the 1960s, 70s, and 80s. This was a time when benzodiazepines were a key area of focus for pharmaceutical companies looking for new blockbuster medications. A lot of these medications didn’t make it past the clinical testing stage and were never approved for medical practice.
For a drug to be approved by the FDA or equivalent regulatory agencies around the world, millions of dollars must first be spent testing the drug through in vitro tests, animal studies, and phase I, II, and III clinical trials.
Drugs like Xanax, Librium, Valium, and Klonopin have all undergone this testing and are not considered research chemicals.
Some substances considered DBZDs aren’t true research chemicals because they’ve been approved by regulatory agencies in a small number of countries; this includes drugs like etizolam and phenazepam.
There are a lot of designer benzodiazepines (DBZDs) on the market today — most of which we’ll cover in more detail below.
The most common DBZDs, according to the European Monitoring Centre for Drugs & Drug Abuse (EMCDDA), include:
These 5 compounds have accounted for as much as 80% of all tablets containing DBZD in Europe since 2005.
Other common DBZDs include:
- Bromazolam
- Clonazolam
- Diclazepam
- Flualprazolam
- Fluclotizolam
- Flunitrazolam
- Flubromazepam
- Flubrotizolam
- Pyrazolam
- Meclonazepam
The safety, efficacy, and metabolism of most of these drugs is unknown.
Benzodiazepine Equivalent Dosage Calculator
This calculator calculates the approximate equivalence between different benzodiazepine derivatives.
Benzo dosage equivalence is not a hard science — there is limited evidence available in the research to compare benzodiazepine equivalence.
This calculator is based on available dosage information for each drug combined with expert opinion.
This dosage does not substitute for expert medical advice. This calculator is intended for informational purposes only.
Caution is advised with higher dosages. Equivalence is less reliable in higher doses or for drugs with longer durations of effects.
**Caution:** Benzodiazepines have a narrow therapeutic window. Dose equivalents may not be accurate in higher doses.
This calculator does not substitute for clinical experience and is meant to serve only as a reference for determining oral benzodiazepine equivalence.
Please consult a medical practitioner before taking benzodiazepines.
Exhaustive List of All Benzodiazepines
There are well over 1500 benzodiazepines in existence. Many of the drugs in use today were invented in the 70s or 80s by companies like Pfizer, Hoffman-La Roche, or Upjohn, but new compounds continue to be discovered every year.
Here, we break down the details for a wide range of benzos — including approved medicines and designer drugs.
1,4-Benzodiazepines
The 1,4-benzodiazepines (1,4-BZDs) are the largest and oldest group of benzodiazepines. Blockbuster medications like Librium, Valium, and Klonopin are all members of this group.
The 1,4-BZD group tends to have a higher affinity for sedative effects than some of the other groups, such as the 1,5-BZDs [63]. The 1,4 group also tends to be very long-lasting in terms of their effects.
2-Oxoquazepam
2-Oxoquazepam was developed in the late 70s but never made it to market. This compound is one of the active metabolites of quazepam (Doral). Both 2-Oxoquazepam and quazepam (Doral) were invented by the Schering Corporation as a treatment for insomnia.
2-Oxoquazepam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 93250 |
| CAS#: | 49606-44-2 |
IUPAC Name: 7-chloro-5-(2-fluorophenyl)-1-(2,2,2-trifluoroethyl)-3H-1,4-benzodiazepin-2-one
3-Hydroxyphenazepam
3-hydroxyphenazepam was first reported in 2016 but hasn’t been approved for medical use. It sometimes appears on designer drug markets.
This potent compound is both the active metabolite of phenazepam and the metabolite of the prodrug cinazepam [64].
3-Hydroxyphenazepam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 125820 |
| CAS#: | 70030-11-4 |
IUPAC Name: 7-Bromo-5-(2-chlorophenyl)-3-hydroxy-1,3-dihydro-2H1,4-benzodiazepin-2-one
BMS-906024
BMS-906024 is an atypical benzodiazepine, which means it shares a similar chemical structure as other benzos but doesn’t work the same way. BMS-906024 is a pan-NOTCH inhibitor — which is useful as an antiproliferative in the treatment of certain kinds of cancers.
This drug was developed by Bristol-Myers Squibb and patented in 2012. It appears this drug is still undergoing clinical testing.
BMS-906024 Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 66550890 |
| CAS#: | 1401066-79-2 |
IUPAC Name: (2S,3R)-N’-[(3S)-1-methyl-2-oxo-5-phenyl-3H-1,4-benzodiazepin-3-yl]-2,3-bis(3,3,3-trifluoropropyl)butanediamide
Bromazepam (Lectopam)
Bromazepam (Lectopam) is one of the most popular street-level benzos available. The effects and dosage of this drug are comparable to Valium, so a lot of people that have prescriptions for Valium revoked or simply can’t afford the high cost of brand-name medications turn to drugs like bromazepam instead.
This compound was invented by Roche in the early 60s but wasn’t approved for medical use until 1974. The patents have since expired, and there are now dozens, if not hundreds, of manufacturers making generic versions of this substance.
Bromazepam Specs:
| Status: | Approved |
| Duration of Effects: | Intermediate-Acting |
| Common Dosage: | 5–10 mg |
| PubChem ID: | 2441 |
| CAS#: | 1812-30-2 |
IUPAC Name: 7-bromo-5-(pyridin-2-yl)-1H-benzo[e][1,4]diazepin-2(3H)-one
Camazepam
Camazepam is sold under the names Albego, Limpidon, and Paxor. It’s the dimethyl carbamate ester of temazepam, and a metabolite of diazepam (Valium).
This compound is different from other benzos in that it maintains strong anxiolytic effects but doesn’t work well as a hypnotic, muscle-relaxant, or intoxicant.
This drug is rarely used in the designer drug space.
Camazepam Specs:
| Status: | Approved |
| Duration of Effects: | Short-Acting |
| Common Dosage: | 10–40 mg |
| PubChem ID: | 37367 |
| CAS#: | 36104-80-0 |
IUPAC Name: 7-chloro-1-methyl-2-oxo-5-phenyl-2,3-dihydro-1H-1,4-benzodiazepin-3-yl N,N-dimethylcarbamate
Carburazepam
There’s very little information about this Russian-made benzodiazepine, but it’s sometimes seen on designer drug marketplaces under the name Uxepam.
One Rusian study concluded that Uxepam was able to remove emotional-behavioral abnormalities under conflict situation, reduces aggressiveness, prevents convulsions, and prolongs the hypnotic effects of other drugs [65]. The study also noted reduced sedative qualities and anxiolytic effects most comparable to chlordiazepoxide.
Carburazepam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 68787 |
| CAS#: | 59009-93-7 |
IUPAC Name: 7-chloro-1-methyl-2-oxo-5-phenyl-3,5-dihydro-1,4-benzodiazepine-4-carboxamide
Chlordiazepoxide (Librium)
Chlordiazepoxide (Librium) was the first benzodiazepine to be approved by the FDA. It was synthesized completely by accident while a chemist was altering the structure of a class of dyes called quinazolone-3-oxides.
The drug was patented in 1958 and approved for medical practice just two years later, in 1960.
The half-life of chlordiazepoxide itself is considered “medium-to-long,” but the active metabolite, nordiazepam (desmethyldiazepam), is very long-lasting.
Chlordiazepoxide Specs:
| Status: | Approved |
| Duration of Effects: | Long-Acting |
| Common Dosage: | 10–20 mg |
| PubChem ID: | 2712 |
| CAS#: | 58-25-3 |
IUPAC Name: 7-chloro-4-hydroxy-N-methyl-5-phenyl-3H-1,4-benzodiazepin-2-imine
Cinazepam
Cinazepam (AKA Levana or BD-798) is an atypical benzodiazepine derivative — meaning it looks like a benzo, but it doesn’t act like one. This compound is only a partial agonist of the GABA-A receptor, and there’s an indication it targets other receptor groups as well.
Levana is considered one of the rare benzodiazepine drugs that don’t disrupt sleep architecture. Slow wave sleep and REM sleep remain proportional when using this drug. For this reason, many doctors prefer using cinazepam for patients with insomnia rather than other benzos that disrupt normal sleep patterns.
Cinazepam Specs:
| Status: | Approved |
| Duration of Effects: | Long-Acting |
| Common Dosage: | Unspecified |
| PubChem ID: | 629281 |
| CAS#: | 172986-25-3 |
IUPAC Name: 4-[[7-bromo-5-(2-chlorophenyl)-2-oxo-1,3-dihydro-1,4-benzodiazepin-3-yl]oxy]-4-oxobutanoic acid
Cinolazepam
Cinolazepam (AKA Gerodorm) was patented in the late 70s during a wave of research among pharmaceutical companies during this time. It took nearly 15 years before the drug was approved for medical use.
This compound was never available in the US or Canada but can still be prescribed in places like Romania.
Cinolazepam is a particularly strong sedative and is only used medically for insomnia.
Cinolazepam Specs:
| Status: | Approved |
| Duration of Effects: | Short-Acting |
| Common Dosage: | 30–60 mg |
| PubChem ID: | 3033621 |
| CAS#: | 75696-02-5 |
IUPAC Name: 3-[7-chloro-5-(2-fluorophenyl)-3-hydroxy-2-oxo-3H-1,4-benzodiazepin-1-yl]propanenitrile
Clonazepam (Klonopin)
Clonazepam (Klonopin) is one of the most popular benzodiazepines on the market. This blockbuster drug was the 46th most prescribed medication in 2019. Google Trend data suggests interest in Klonopin is only continuing to grow. This compound is one of the easiest to find on designer drug markets.
A lot of young people are abusing Klonopin for its intoxicating effects. It’s considered more euphoric, more intoxicating, and less sedative than many of the other members of this class.
Clonazepam was first patented in 1960 by Roche, so the patents have long since expired. Many different companies now produce this drug on a commercial scale.
Clonazepam is 1,3-Dihydro-2H-1,4-benzodiazepin-2-one in which the hydrogens at positions 5 and 7 are substituted by 2-chlorophenyl and nitro groups, respectively.
Clonazepam Specs:
| Status: | Approved |
| Duration of Effects: | Intermediate-Acting |
| Common Dosage: | 0.5–1 mg |
| PubChem ID: | 2802 |
| CAS#: | 1622-61-3 |
IUPAC Name: 5-(2-chlorophenyl)-7-nitro-1,3-dihydro-1,4-benzodiazepin-2-one
Cloniprazepam
Cloniprazepam was first reported in 2016 as a research chemical. The creator of this drug remains unknown.
Unsurprisingly, very little research is available for this drug, but it’s suspected to be a prodrug of clonazepam (Klonopin) — meaning that once you take cloniprazepam it’s converted to clonazepam (Klonopin) by the liver.
Other experts suggest this compound is a prodrug of other benzos, including 7-aminoclonazepam, 3-hydroxyclonazepam, 6-hydroxyclonazepam, 3-hydroxycloniprazepam, and ketocloniprazepam.
Cloniprazepam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 137700140 |
| CAS#: | 1998158-84-1 |
IUPAC Name: 5-(2-Chlorophenyl)-1-(cyclopropylmethyl)-7-nitro-1,3-dihydro-2H-benzo[e][1,4]diazepin-2-one
Clorazepate
Clorazepate (Tranxene) is one of the few water-soluble benzodiazepines. This alters bioavailability in the gut (thought to improve absorption) and brain (thought to reduce passage across the blood-brain barrier).
This compound is unusually long-lasting. As it’s metabolized, clorazepate is metabolized into desmethyldiazepam, which also has an abnormally long half-life.
This drug was invented in the 1960s and approved in 1967. Today it’s primarily used in the treatment of alcohol withdrawal syndrome due to the long duration of effects.
Clorazepate Specs:
| Status: | Approved |
| Duration of Effects: | Long-Acting |
| Common Dosage: | 10–20 mg |
| PubChem ID: | 2809 |
| CAS#: | 23887-31-2 |
IUPAC Name: 7-chloro-2-oxo-5-phenyl-1,3-dihydro-1,4-benzodiazepine-3-carboxylic acid
Cyprazepam
Very little information is available about this drug. It hasn’t been tested for safety, and the pharmacology is only hypothesized by its similarities to other members of the 1,4-BZD group.
Cuprazepam is believed to be a strong hypnotic with anxiolytic effects.
This drug has not yet appeared on any designer drug marketplaces in the United States, and it’s unclear if people are using it for its intoxicating effects.
Cyprazepam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 27452 |
| CAS#: | 15687-07-7 |
IUPAC Name: 7-chloro-N-(cyclopropylmethyl)-4-hydroxy-5-phenyl-3H-1,4-benzodiazepin-2-imine
Delorazepam
Delorazepam (Dadumir) is sold in Italy for the treatment of anxiety and insomnia. It’s one of the few naturally-occurring benzodiazepines and is surprisingly potent. This compound is roughly ten times as strong as diazepam and has a high bioavailability of nearly 80%.
This drug can be extracted from Solanum tuberosum, Triticum aestivum, and Artemisia dracunculus.
Delorazepam is an analog of desmethyldiazepam and an active metabolite of diclazepam and cloxazolam.
Delorazepam Specs:
| Status: | Approved (Italy) |
| Duration of Effects: | Long-Acting |
| Common Dosage: | Unspecified |
| PubChem ID: | 17925 |
| CAS#: | 2894-67-9 |
IUPAC Name: 7-chloro-5-(2-chlorophenyl)-1,3-dihydro-1,4-benzodiazepin-2-one
Demoxepam
The potency and safety of this compound are not well understood. Demoxepam is not a popular research chemical and has never been approved for medical use in any country.
Demoxepam is both a metabolite of chlordiazepoxide and an intermediate in the production of oxazepam.
Demoxepam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Intermediate-Acting |
| Common Dosage: | Unspecified |
| PubChem ID: | 13756 |
| CAS#: | 963-39-3 |
IUPAC Name: 7-chloro-4-hydroxy-5-phenyl-3H-1,4-benzodiazepin-2-one
Desmethylflunitrazepam
Desmethylflunitrazepam (AKA norflunitrazepam, Ro-4435, and fonazepam) is the active metabolite of flunitrazepam — one of the most powerful benzodiazepines in the 1,4-BZD class.
Desmethylflunitrazepam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | 5–15 mg |
| PubChem ID: | 520217 |
| CAS#: | 2558-30-7 |
IUPAC Name: 5-(2-fluorophenyl)-7-nitro-1,3-dihydro-2H-1,4-benzodiazepin-2-one
Devazepide
Devazepide is non-psychoactive.
This compound is an atypical benzodiazepine and does not share the general hypnotic, anxiolytic, or muscle-relaxant qualities of other benzos. This one acts as a cholecystokinin (CCK) antagonist that could be used for treating gastrointestinal disorders. This effect increases appetite and speeds the emptying of the stomach [66].
Devazepide has never been approved for medical use and is not used in the designer drug community.
Devazepide Specs:
| Status: | Approved |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 443375 |
| CAS#: | 103420-77-5 |
IUPAC Name: N-[(3S)-1-methyl-2-oxo-5-phenyl-3H-1,4-benzodiazepin-3-yl]-1H-indole-2-carboxamide
Diazepam
Diazepam (Valium) was the first blockbuster benzodiazepine. It was the best-selling medication in the United States for nearly 15 years, between 1968 and 1982. More than 2 billion tablets of Valium were sold in 1978 alone [14].
Today, Diazepam has fallen out of favor to other drugs like alprazolam (#1 prescribed benzodiazepine), clonazepam (#2), and lorazepam (#3).
Diazepam was patented in 1959 by Hoffmann-La Roche and put on the market officially in 1963. At the time, diazepam was a clear improvement to the previous generation of hypnotic anxiolytics — the barbiturates. The effects were also considered an improvement on its progenitor, Librium.
This drug is classified as a long-acting “classical” benzodiazepine in the 1,4-BZD group. It’s most closely related to other classical benzos such as chlordiazepoxide, clonazepam, lorazepam, oxazepam, nitrazepam, temazepam, flurazepam, bromazepam, and clorazepate.
Diazepam is often considered the benchmark for assessing the potency of other benzodiazepines. Potencies are generally assessed by the equivalent dose is to 10 mg of diazepam.
The patent has long expired for this medication, so there are now hundreds of generic options on the market.
Diazepam Specs:
| Status: | Approved |
| Duration of Effects: | Long-Acting |
| Common Dosage: | 5–25 mg |
| PubChem ID: | 3016 |
| CAS#: | 439-14-5 |
IUPAC Name: 7-chloro-1-methyl-5-phenyl-3H-1,4-benzodiazepin-2-one
Diclazepam
Like many research benzodiazepines, diclazepam was invented in the 1960s by Hoffman-La Roche but never made it to medical practice. It appeared just ten years ago on the designer drug market.
People who use this drug like the long duration of effects — the elimination half-life for this drug is 42 hours, which is substantially higher than average.
Diclazepam is a functional analog of diazepam. It’s metabolized by the liver into lorazepam and lormetazepam, which remain active for several hours.
Other names for this drug include 2-Chlorodiazepam and RO5-3448.
Diclazepam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Intermediate-Acting |
| Common Dosage: | 1–2 mg |
| PubChem ID: | 76168 |
| CAS#: | 2894-68-0 |
IUPAC Name: 7-chloro-5-(2-chlorophenyl)-1-methyl-3H-1,4-benzodiazepin-2-one
Difludiazepam
Difludiazepam (RO07-4065) is the 2,6-difluoro derivative of fludiazepam — one of the most potent benzodiazepines currently available. The addition of an extra fluoride group to this molecule is thought to enhance the bioavailability substantially — leading to much higher potency than its already powerful precursor.
This drug was invented in the 70s but never marketed. It’s since showed up in the designer drug space a few years ago, around 2016.
Difludiazepam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 44366236 |
| CAS#: | 39080-67-6 |
IUPAC Name: 7-chloro-5-(2,6-difluorophenyl)-1-methyl-3H-1,4-benzodiazepin-2-one
Doxefazepam (Doxans)
Doxefazepam (Doxans) is a potent derivative of flurazepam. This drug is believed to be somewhere between 2 and 4 times as potent as its progenitor while possessing roughly half the toxicity (based on animal testing) [16].
This drug was patented in 1972 and entered medical use 12 years later in 1984. The patents have since expired, so there are a number of companies producing generic versions of this drug today.
The elimination half-life of docefazepam is short — just 3 or 4 hours total.
Doxefazepam Specs:
| Status: | Approved |
| Duration of Effects: | Short-Acting |
| Common Dosage: | Unspecified |
| PubChem ID: | 38668 |
| CAS#: | 40762-15-0 |
IUPAC Name: 7-chloro-5-(2-fluorophenyl)-3-hydroxy-1-(2-hydroxyethyl)-3H-1,4-benzodiazepin-2-one
Elfazepam
Elfazepam has qualities of a classical benzo (hypnotic and anxiolytic) as well as some of the more atypical benzos (orexigenic and gastric acid secretion inhibition) [17,18].
This drug was never used in medicine and is not popular as a recreational drug.
Elfazepam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 65445 |
| CAS#: | 52042-01-0 |
IUPAC Name: 7-chloro-1-(2-ethylsulfonylethyl)-5-(2-fluorophenyl)-3H-1,4-benzodiazepin-2-one
Ethyl Carfluzepate
Ethyl carfluzepate is structurally similar to ethyl loflazepate, with the only difference being an absence of a methylcarbamoyl group.
Not much information is available for either of these drugs, so it’s unclear how this subtle difference changes the drug’s effects. However, ethyl loflazepate is known to be very strong and very long-lasting — providing all classical benzodiazepine effects (hypnotic, sedative, anxiolytic, intoxicant, muscle-relaxant, etc.).
Ethyl Carfluzepate Specs:
| Status: | Research Chemical |
| Duration of Effects: | Intermediate-Acting |
| Common Dosage: | Unspecified |
| PubChem ID: | 68856 |
| CAS#: | 65400-85-3 |
IUPAC Name: ethyl 7-chloro-5-(2-fluorophenyl)-1-(methylcarbamoyl)-2-oxo-3H-1,4-benzodiazepine-3-carboxylate
Ethyl Dirazepate
Ethyl Dirazepate was developed by Sanofi Winthrop but never taken to market. The specific effect profile of this drug remains unknown.
Ethyl dirazepate Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 208941 |
| CAS#: | 23980-14-5 |
IUPAC Name: ethyl 7-chloro-5-(2-chlorophenyl)-2-oxo-1,3-dihydro-1,4-benzodiazepine-3-carboxylate
Ethyl Loflazepate (Meilax)
Ethyl Loflazepate (Meilax, Ronlax, and Victan) is a prodrug for another benzodiazepine called descarboxyloflazepate which is much stronger than ethyl loflazepate [19]. Other metabolites include loflazepate and 3-hydroxydescarbethoxyloflazepate, both of which are believed to be active as well [20].
The elimination half-life of this drug is extremely long (50–100 hours).
Ethyl loflazepate Specs:
| Status: | Approved |
| Duration of Effects: | Long-Acting |
| Common Dosage: | Unspecified |
| PubChem ID: | 3299 |
| CAS#: | 29177-84-2 |
IUPAC Name: ethyl 7-chloro-5-(2-fluorophenyl)-2-oxo-1,3-dihydro-1,4-benzodiazepine-3-carboxylate
Fletazepam
Fletazepam is an especially potent muscle-relaxant but maintains moderate anxiolytic and hypnotic qualities as well [67].
Structurally, fletazepam is similar to both halazepam and quazepam. All three drugs are classified as N-triflyouroethyl substituted benzodiazepines.
Fletazepam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 36834 |
| CAS#: | 34482-99-0 |
IUPAC Name: 7-chloro-5-(2-fluorophenyl)-1-(2,2,2-trifluoroethyl)-2,3-dihydro-1,4-benzodiazepine
Flubromazepam
Flubromazepam was first synthesized in 1960 but never studied further. It wasn’t until over 50 years later that it first started popping up in drug detection databases in the US and Europe.
This compound is a structural analog of phenazepam. The difference is the replacement of a chlorine atom from the phenazepam molecule with fluorine in flubromazepam. The addition of fluorine to psychoactive drugs often leads to much higher bioavailability and potency of effects.
An alternate isomer, iso-flubromazepam is sometimes sold under the same name [21].
Flubromazepam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Long-Acting |
| Common Dosage: | 4–8 mg |
| PubChem ID: | 12947024 |
| CAS#: | 2647-50-9 |
IUPAC Name: 7-Bromo-5-(2-fluorophenyl)-1,3-dihydro-2H-1,4-benzodiazepin-2-one
Fludiazepam (Erispan)
Fludiazepam (Erispan) was developed in the 1960s by Hoffman-La Roche. It was approved and marketed in both Japan and Taiwan but was never approved in the US or European Union.
This compound is the 2ʹ-fluoro derivative of diazepam and shares similar effects but nearly four times the potency of this classic benzodiazepine [22].
There is evidence this drug has been used recreationally in Japan and other Asian countries since at least the early 90s [23].
Fludiazepam Specs:
| Status: | Approved |
| Duration of Effects: | Intermediate-Acting |
| Common Dosage: | Unspecified |
| PubChem ID: | 3369 |
| CAS#: | 3900-31-0 |
IUPAC Name: 7-chloro-5-(2-fluorophenyl)-1-methyl-3H-1,4-benzodiazepin-2-one
Flunitrazepam (Rohypnol)
Flunitrazepam (Rohypnol) is one of the only members of the benzodiazepine family that’s classified as a Schedule III controlled substance in the US. Most other benzodiazepines (both approved and not-approved) are grouped into the Schedule IV tier instead.
What this means is that the FDA deems this drug to have a higher potential for abuse than the vast majority of other benzodiazepines. The more common name for this drug is Rohypnol — which is most often abused as a date rape drug (called Roofies or Floonies).
You can only get this medication via prescription, and there are several extra layers of verification before patients can gain access to it as a medicfation. For this reason, in combination with its high risk of abuse, flunitrazepam is rarely prescribed today.
Structurally, flunitrazepam is a 1,4-benzodiazepinone, a C-nitro compound, and a member of monofluorobenzenes. It’s essentially a nitrazepam with a fluoro group at position 2 and methyl group in position one. The addition of fluoride groups has a tendency to increase the potency of drugs. This appears to be the case for flunitrazepam compared to its progenitor, nitrazepam.
Flunitrazepam Specs:
IUPAC Name: 5-(2-fluorophenyl)-1-methyl-7-nitro-3H-1,4-benzodiazepin-2-one
Flurazepam (Dalmane)
Flurazepam (Dalmane and Dalmadorm) was one of the first sleeping pills (benzo hypnotics) to be marketed in the United States. This drug was developed by Roche in the late 1960s.
Structurally, flurazepam is a 1,4-benzodiazepinone, organochlorine compound, and a member of monofluorobenzenes.
This drug has a particularly long half-life, remaining in the bloodstream for several days.
Flurazepam Specs:
| Status: | Approved |
| Duration of Effects: | Long-Acting |
| Common Dosage: | 15–30 mg |
| PubChem ID: | 3393 |
| CAS#: | 17617-23-1 |
IUPAC Name: 7-chloro-1-[2-(diethylamino)ethyl]-5-(2-fluorophenyl)-3H-1,4-benzodiazepin-2-one
Flutemazepam
Flutemazepam was developed by Stabilimenti Chimici Farmaceutici Riuniti SpA — an Italian pharmaceutical company that’s no longer in bisiness. Much like most of the other benzos, this compound was developed around the mid-1970s.
This compound is an analog of temazepam, which is sold under the brand name Restoril.
Flutemazepam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 40344 |
| CAS#: | 52391-89-6 |
IUPAC Name: 7-chloro-5-(2-fluorophenyl)-3-hydroxy-1-methyl-3H-1,4-benzodiazepin-2-one
Flutoprazepam (Restas)
Flutoprazepam (Restas) was first patented in 1972 by the Japanese pharmaceutical company Simitomo in 1972. It’s been shown to be around four times more potent than diazepam [24].
The active metabolite of this drug, n-desalkylflurazepam (AKA norflurazepam) is also the primary metabolite of flurazepam (Dalmane) [25].
Flutoprazepam Specs:
| Status: | Approved |
| Duration of Effects: | Long-Acting |
| Common Dosage: | 1.5–3 mg |
| PubChem ID: | 3400 |
| CAS#: | 25967-29-7 |
IUPAC Name: 7-chloro-1-(cyclopropylmethyl)-5-(2-fluorophenyl)-3H-1,4-benzodiazepin-2-one
Fosazepam
Fosazepam is the water-soluble derivative of diazepam, thanks to the addition of a dimethylphosphoryl group. It’s roughly 10% as strong as nitrazepam, and about 20% as potent as diazepam [26].
The effects of this drug are most similar to nitrazepam, but most people consider it to be milder and shorter-acting. Because of its more gentle nature, fosazepam is less likely to lead to side effects such as lethargy, sedation, cognitive impairments, memory loss, rebound insomnia, and brain fog [27].
Fosazepam Specs:
| Status: | Research Chemical |
| Duration of Effects: | 12+ hours |
| Common Dosage: | 2–20 mg |
| PubChem ID: | 37114 |
| CAS#: | 35322-07-7 |
IUPAC Name: 7-chloro-1-(dimethylphosphorylmethyl)-5-phenyl-3H-1,4-benzodiazepin-2-one
Gidazepam
Gidazepam (AKA hydazepam or hidazepam) was developed in the Soviet Union in the early 90s. It’s a prodrug for the active metabolite 7-bromo-5-phenyl-1,2-dihydro-3H-1,4-benzodiazepine-2-one (desalkylgidazepam) [28,29].
The half-life of this drug is exceptionally long, but no specific timeframe has ever been examined. It’s likely this drug remains active for around 12–24 hours.
Gidazepam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Long-Acting |
| Common Dosage: | Unspecified |
| PubChem ID: | 121919 |
| CAS#: | 129186-29-4 |
IUPAC Name: 2-(7-bromo-2-oxo-5-phenyl-3H-1,4-benzodiazepin-1-yl)acetohydrazide
Halazepam (Paxipam)
Halazepam is sold under several different names. In the US it’s sold as Paxipam; in Spain, Alapryl; and in Portugal, Pacinone.
This drug was invented by Schlesinger Walter and marketed in the early 80s. It was sold as being “less toxic” than chlordiazepoxide (Librium) and diazepam (Valium) but was later pulled due to abysmal sales.
Halazepam Specs:
| Status: | Approved (Discontinued) |
| Duration of Effects: | Long-Acting |
| Common Dosage: | 20–40 mg |
| PubChem ID: | 31640 |
| CAS#: | 23092-17-3 |
IUPAC Name: 7-chloro-5-phenyl-1-(2,2,2-trifluoroethyl)-3H-1,4-benzodiazepin-2-one
Iclazepam
Iclazepam is a derivative and prodrug of nordazepam. The potency of this drug is believed to be comparable to chlordiazepoxide (Librium), but not much research is available to elucidate this.
Iclazepam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 68777 |
| CAS#: | 57916-70-8 |
IUPAC Name: 7-chloro-1-[2-(cyclopropylmethoxy)ethyl]-5-phenyl-3H-1,4-benzodiazepin-2-one
Irazepine
Irazepine (Ro7-1986/1) was under development by Roche but never made it to market. This compound is characterized by the presence of a isothiocyanate functional group.
Unlike other benzodiazepines, irazepine acts as a non-competitive GABA-A antagonist [68] (blocks the effects of benzodiazepines).
Irazepine Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 194406 |
| CAS#: | 71735-92-7 |
IUPAC Name: 7-chloro-5-(2-fluorophenyl)-1-(2-isothiocyanatoethyl)-3H-1,4-benzodiazepin-2-one
Ketazolam (Anxon)
Ketazolam is sold under a variety of brand names, including Anseren, Ansieten, Ansietil, Anxon, Marcen, Sedatival, Sedotime, Solatran, and Unakalm. It’s one of the more popular options in medical practice in recent years because of its reduced risk and severity of side effects (especially hypnotic side effects) compared to conventional options like diazepam [30]. This drug is mainly used as a muscle relaxant.
Ketazolam Specs:
| Status: | Approved |
| Duration of Effects: | Long-Acting |
| Common Dosage: | 15–30 mg |
| PubChem ID: | 33746 |
| CAS#: | 27223-35-4 |
IUPAC Name: 11-chloro-2,8-dimethyl-12b-phenyl-6H-[1,3]oxazino[3,2-d][1,4]benzodiazepine-4,7-dione
Lorazepam (Ativan)
Lorazepam (Ativan) was patented in 1963 by Wyeth Pharmaceuticals and went on sale in 1977. It’s one of the few benzodiazepines listed on the World Health Organization’s List of Essential Medicines.
Lorazepam is used for all the usual benzodiazepine applications — insomnia, anxiety, muscle tension — as well as an amnesic during surgery. Ativan has a marked effect on memory inhibition, which is also one of the drug’s most problematic side effects.
The patent for this drug has expired, so there are hundreds of companies producing generic versions today.
Lorazepam Specs:
| Status: | Approved |
| Duration of Effects: | Intermediate-Acting |
| Common Dosage: | 0.5–2 mg |
| PubChem ID: | 3958 |
| CAS#: | 846-49-1 |
IUPAC Name: 7-chloro-5-(2-chlorophenyl)-3-hydroxy-1,3-dihydro-1,4-benzodiazepin-2-one
Lormetazepam (Noctamid)
Lormetazepam (Noctamid) was invented in 1961 but didn’t enter medical use until nearly 20 years later, in 1980. The patents for this drug have long worn out, so there are many generic options on the market today.
This drug is primarily used as a sedative and rarely for its intoxicating or anxiolytic effects. Most people who take this drug just fall asleep, so it isn’t considered “recreational” in any particular way.
The elimination half-life is shorter than many other benzodiazepines, which makes it a good candidate as a sleep aid that doesn’t cause a great deal of drowsiness the following morning.
Lormetazepam features a methyl group in the 1-position, a hydroxy group in the 3-position, a 2-chlorophenyl group in the 5-position, and a chloride group in the 7-position.
Lormetazepam Specs:
IUPAC Name: 7-chloro-5-(2-chlorophenyl)-3-hydroxy-1-methyl-3H-1,4-benzodiazepin-2-one
Lufuradom
Lufuradom is an atypical benzodiazepine drug used as an analgesic rather than the conventional anxiolytic, hypnotic, and muscle-relaxant.
This compound is an active analog of another benzo called tifluadom. It was never marketed and is not well used in the grey market today due to the lack of intoxicating effects.
Lufuradom Specs:
| Status: | Not Used |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 3045400 |
| CAS#: | 94006-14-1 |
IUPAC Name: N-[(8-fluoro-1-methyl-5-phenyl-2,3-dihydro-1,4-benzodiazepin-2-yl)methyl]furan-3-carboxamide
Meclonazepam
Meclonazepam is a research chemical first reported on the grey market in 2014. It was discovered much earlier than this, around the early 1970s, by the research team at Hoffmann-La Roche.
The chemical structure of this compound is similar to clonazepam (Klonopin), but the effect profile is distinct.
What makes meclonazepam different from other benzos is its unique antiparasitic effect. This action was found to be particularly effective against the parasitic worm Schistosoma mansoni [31].
Meclonazepam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 3033985 |
| CAS#: | 58662-84-3 |
IUPAC Name: (3S)-5-(2-Chlorophenyl)-3-methyl-7-nitro-1,3-dihydro2H-1,4-benzodiazepin-2-one
Medazepam (Nobrium)
Medazepam is a prodrug for both diazepam, as well as oxazepam, nordiazepam, and temazepam — so the effects are comparable to these other, more prevalent compounds.
This drug is extremely long-lasting — the elimination half-life of medazepam ranges from 36 to 200 hours — so the effects can last anywhere from a full day to three days in people with slower phase I and II liver metabolism.
Medazepam is sold under the brand names Azepamid, Nobrium, Tranquirax, Rudotel, Raporan, Ansilan, Mezapam, and numerous others.
Medazepam Specs:
IUPAC Name: 7-chloro-1-methyl-5-phenyl-2,3-dihydro-1,4-benzodiazepine
Menitrazepam
The structure of Menitrazepam is similar to tetrazepam and nimetazepam. The difference is the addition of a nitro group to the menitrazepam structure.
Not much research is available to assess the difference this nitro group makes on the molecule — but several other benzos with a nitro group attached are noted to be particularly potent.
Menitrazepam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 189875 |
| CAS#: | 28781-64-8 |
IUPAC Name: 5-(cyclohexen-1-yl)-1-methyl-7-nitro-3H-1,4-benzodiazepin-2-one
Metaclazepam (Talis)
Metaclazepam is sold as Talis and prescribed for anxiety and as a muscle relaxant.
The active metabolite of metaclazepam is N-desmethylmetaclazepam [32].
As is the case with many benzodiazepines, the activity of these drugs will vary as the compounds are metabolized. This makes it hard to predict the optimal dose or effect outcome in different people.
Metaclazepam is considered slightly more effective for managing anxiety symptoms than the more popular benzos, bromazepam, or diazepam.
Metaclazepam Specs:
| Status: | Approved |
| Duration of Effects: | Short-Acting |
| Common Dosage: | 10–20 mg |
| PubChem ID: | 71272 |
| CAS#: | 84031-17-4 |
IUPAC Name: 7-bromo-5-(2-chlorophenyl)-2-(methoxymethyl)-1-methyl-2,3-dihydro-1,4-benzodiazepine
N-Desalkylflurazepam (Norflurazepam)
N-Desalkylflurazepam (norflurazepam) is unlike other benzodiazepines in that it binds to both the BZ1 and BZ2 receptor sites on the GABA-A receptor [33].
This compound is approved as a medicine but is the active metabolite of several other medications and research chemicals — including ethyl loflazepate, fludiazepam, flurazepam, flutoprazepam, flutazolam, midazolam, quazepam, and potentially others.
Pure N-desalkylflurazepam has been appearing on the designer drug market since at least 2017 [34]. It’s known for its long-lasting effects but is also prone to problems associated with accumulation.
N-Desalkylflurazepam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 4540 |
| CAS#: | 2886-65-9 |
IUPAC Name: 7-chloro-5-(2-fluorophenyl)-1,3-dihydro-1,4-benzodiazepin-2-one
Nifoxipam
Nifoxipam (3-hydroxydesmethylflunitrazepam) is one of the metabolites of flunitrazepam (minor) but is also sold on its own. Some grey market vendors are selling this drug under the name DP370.
The effects of this drug are comparable to both lormetazepam and flunitrazepam, but there are reports the risk of side effects is lower with nifoxipam. This has not yet been confirmed through clinical research.
Nifoxipam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 3058221 |
| CAS#: | 74723-10-7 |
IUPAC Name: 5-(2-Fluorophenyl)-3-hydroxy-7-nitro-2,3-dihydro-1H1,4-benzodiazepin-2-one
Nimetazepam (Lavol)
Nimetazepam (Erimin and Lavol) is structurally similar to nitrazepam (Mogadon and Alodorm) — the only difference is the addition of a methyl group in position one of the molecule. The effects of this drug are very similar to other nitrazepam analogs, such as flunitrazepam.
This substance is considered an intermediate-acting hypnotic with a particularly rapid onset of effects. The effects of nimetazepam can be felt in as little as 15 minutes after oral ingestion.
This drug was first invented in 1964 by Hoffmann-La Roche but was exclusively manufactured by the Japanese pharmaceutical company Sumitomo in the 80s and 90s. The drug maker is no longer producing this drug, but it remains abundant on grey markets throughout Southeast Asia.
There are also reports that nimetazepam is a common adulterant in Blackmarket methamphetamine, MDMA, and opiate samples in SEA.
Nimetazepam Specs:
| Status: | Approved |
| Duration of Effects: | Intermediate-Acting |
| Common Dosage: | Unspecified |
| PubChem ID: | 4496 |
| CAS#: | 2011-67-8 |
IUPAC Name: 1-methyl-7-nitro-5-phenyl-3H-1,4-benzodiazepin-2-one
Nitemazepam
Nitemazepam (3-hydroxynimetazepam) was first made in the 70s but never made it to market. It’s a prodrug of several other benzodiazepines, including nimetazepam, and temazepam. Other active metabolites include 3-hydroxynitemazepam, 7-aminonitemazepam, and nimetazepam glucuronide [11].
This compound is the 7-nitro analog of temazepam (contains a 7-chloro group instead).
Nitemazepam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Intermediate-Acting |
| Common Dosage: | Unspecified |
| PubChem ID: | 12362353 |
| CAS#: | 40762-03-6 |
IUPAC Name: 3-Hydroxy-1methyl-7-nitro-5-phenyl-2,3-dihydro-1H1,4-benzodiazepin-2-one
Nitrazepam (Mogadon)
Nitrazepam (Mogadon) is a nitro-containing benzodiazepine derivative. The addition of a nitro group often increases potency and sometimes enhances the metabolic half-life. Only the first is the case for nitrazepam, which is roughly twice as potent as diazepam but shares a comparable duration of effects (half-life is between 16.5 and 48.3 hours).
Interestingly, nitrazepam has been found to dramatically increase growth hormone in humans [106].
This compound was patented in 1961 and entered medical use four years later in 1965.
Nitrazepam Specs:
| Status: | Approved |
| Duration of Effects: | Intermediate-Acting |
| Common Dosage: | 5–15 mg |
| PubChem ID: | 4506 |
| CAS#: | 146-22-5 |
IUPAC Name: 7-nitro-5-phenyl-1,3-dihydro-1,4-benzodiazepin-2-one
Nordiazepam (Nordaz)
Nordazepam (AKA nordiazepam, desoxydemoxepam, and desmethyldiazepam) is sold under the trade names Nordaz, Stilny, Madar, Vegesan, and Calmday.
This 1,4-benzodiazepine derivative is one of the few naturally-occurring benzodiazepines on Earth. It’s been found in soybeans (Glycine max) and maize corn (Zea mays) — both in minuscule quantities [109].
This compound is defined structurally by the presence of phenyl and chloro functional groups in the 5 and 7 positions, respectively.
This drug is one of the longest-lasting benzos available. The elimination half-life sits somewhere between 36 and 200 hours. One of the reasons for this is that both the drug itself and its active metabolite, oxazepam, both have a long half-life.
Nordazepam Specs:
IUPAC Name: 7-chloro-5-phenyl-1,3-dihydro-1,4-benzodiazepin-2-one
Nortetrazepam
Nortetrazepam isn’t used on its own but is one of the primary active metabolites of tetrazepam.
Sometimes this compound can be found on designer drug markets, but it is not recommended. Tetrazepam, its prodrug, used to be an approved medication but was pulled after it became clear the drug was causing immunological toxicity.
Nortetrazepam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 166581 |
| CAS#: | 10379-11-0 |
IUPAC Name: 7-chloro-5-(cyclohexen-1-yl)-1,3-dihydro-1,4-benzodiazepin-2-one
Oxazepam (Serax)
Oxazepam (Serax) is a smaller, simpler benzodiazepine that forms as a metabolic byproduct for several approved medications, including diazepam, prazepam, and temazepam.
This compound is considered a short-to-intermediate-acting benzo (5–6 hour half-life) with low potency (about half as potent as diazepam).
This drug was used extensively in the 1960s for treating anxiety, insomnia, and alcohol withdrawal. Today it’s been cast aside in favor of stronger, faster-acting, or longer-lasting drugs instead, but it remains relatively popular as a designer drug.
Oxazepam Specs:
| Status: | Approved |
| Duration of Effects: | Intermediate-Acting |
| Common Dosage: | 10–30 mg |
| PubChem ID: | 4616 |
| CAS#: | 604-75-1 |
IUPAC Name: 7-chloro-3-hydroxy-5-phenyl-1,3-dihydro-1,4-benzodiazepin-2-one
Phenazepam
Phenazepam was the first designer benzodiazepine (DBZD) to enter the market. This compound has been on the market since at least 2007 but is believed to have been created in the Soviet Union in the 1970s.
Trade names for this drug include BD98, Elzepam, Fenazepam, Phenazef, Phenazepam, Phezipam, Phenorelaxan, and Trankvezipam.
Phenazepam is well-established on the designer drug market at this point and goes by many street names. A few examples include Bonsai, Bonsai Supersleep, Fenaz, Soviet Benzo, and Panda.
Phenazepam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Long-Acting |
| Common Dosage: | 1–2 mg |
| PubChem ID: | 40113 |
| CAS#: | 51753-57-2 |
IUPAC Name: 7-Bromo-5-(2-chlorophenyl)-1,3-dihydro-2H-1,4-benzodiazepin-2-one
Pinazepam
Pinazepam (Domar and Duna) is of interest medically because of its lack of intellectual, motor, and hypnotic impairing activity [36]. These qualities make pinazepam more appropriate than other benzodiazepines for daytime use. It’s also been suggested to be less toxic in higher doses than diazepam (animal studies).
This compound is characterized by the presence of a propargyl group at the N-1 position of the central benzodiazepine structure.
Pinazepam Specs:
| Status: | Approved |
| Duration of Effects: | Long-Acting |
| Common Dosage: | Unspecified |
| PubChem ID: | 40391 |
| CAS#: | 52463-83-9 |
IUPAC Name: 7-chloro-5-phenyl-1-prop-2-ynyl-3H-1,4-benzodiazepin-2-one
Pivoxazepam
Pivoxazepam is the pivalate (2,2-dimethylpropanoate) ester of oxazepam — another well-known benzodiazepine that’s been on the market since the 60s.
Compared to the parent drug, oxazepam, pivoxazepam is absorbed more quickly and has slightly less sedative qualities (subjective).
Pivoxazepam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 68722 |
| CAS#: | 55299-10-0 |
IUPAC Name: (7-chloro-2-oxo-5-phenyl-1,3-dihydro-1,4-benzodiazepin-3-yl) 2,2-dimethylpropanoate
Prazepam
Prazepam (Lysanxia, Demetrin, Centrax, Prazene, and others) was developed in the 1960s by Warner-Lambert, an American pharmaceutical company that was ultimately bought by Pfizer.
This compound serves as a prodrug for desmethyldiazepam (nordiazepam) and 3-hydroxyprazepam — both of which are further metabolized into oxazepam.
Prazepam itself has little to no effect, so the pharmacological profile of this substance is due to the formation of active metabolites instead. It has a slow onset of effects but a long half-life, up to 224 hours.
Prazepam was found to be one of the top 5 most commonly prescribed benzodiazepines in France [110].
Prazepam Specs:
IUPAC Name: 7-chloro-1-(cyclopropylmethyl)-5-phenyl-3H-1,4-benzodiazepin-2-one
QH-II-66
QH-II-66 is highly selective for the α5 subtype of GABA-A receptors [111]. This subtype is one of the primary targets of alcohol, so this drug is thought to be particularly useful for treating the withdrawal symptoms of alcohol. It’s also believed to have less sedative activities than diazepam or triazolam because of this effect.
The affinity for the α5 subtype also gives QH-II-66 particularly intoxicating effects, which are often compared to the intoxication produced by alcohol. The α5 subtype is also thought to be the primary cause of the amnesic effects of benzodiazepines, but it’s unclear whether QH-II-66 is more amnesic than other benzos or not.
QH-II-66 Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 9838431 |
| CAS#: | 183239-39-6 |
IUPAC Name: 7-ethynyl-1-methyl-5-phenyl-3H-1,4-benzodiazepin-2-one
Quazepam (Doral)
Quazepam (Doral) was developed by the Schering Corporation in the 1970s and later approved as medicine in 1985. It was alleged to maintain sleep without causing disruption of the sleep architecture [37]. This made quazepam a popular option for treating insomnia.
The mechanism of action for this drug is very similar to the modern Z-drugs, zolpidem, and zaleplon.
Quazepam is metabolized into 2-oxoquazepam (active) and N-desalkyl-2-oxoquazepam (low activity). The elimination half-life sits somewhere between 27 and 41 hours total [38].
Structurally, quazepam is considered a trifluoroalkyl type benzodiazepine.
Quazepam Specs:
| Status: | Approved |
| Duration of Effects: | Long-Acting |
| Common Dosage: | 10–15 mg |
| PubChem ID: | 4999 |
| CAS#: | 36735-22-5 |
IUPAC Name: 7-chloro-5-(2-fluorophenyl)-1-(2,2,2-trifluoroethyl)-3H-1,4-benzodiazepine-2-thione
Reclazepam
Not much research is available on this drug. A patent was filed for reclazepam in 1979 for reclazepam by G.D. Searle, LLC, a subsidiary of Pfizer, but nothing ever came of it.
Very little anecdotal data exists for this drug as well, and it doesn’t appear to be a popular research benzodiazepine either.
Reclazepam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 3052777 |
| CAS#: | 76053-16-2 |
IUPAC Name: 2-[7-chloro-5-(2-chlorophenyl)-2,3-dihydro-1,4-benzodiazepin-1-yl]-1,3-oxazol-4-one
RO4491533
RO4491533 was developed by Hoffmann-La Roche in the 1980s during a time when the company was investing heavily in new benzo derivatives.
This atypical benzo doesn’t act strongly, if at all, on the GABA-A receptors. Instead, RO4491533 acts on group II of the metabotropic glutamate receptors as a selective negative allosteric modulator. This action is thought to make the compound more useful as an antidepressant than an anxiolytic or sedative — but no clinical testing was ever done to determine the effects of this substance on humans.
Due to the atypical nature of this drug, it’s not likely to be of interest as a recreational drug.
RO4491533 Specs:
| Status: | Not Used |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 11158623 |
| CAS#: | 579482-31-8 |
IUPAC Name: 4-[3-(2,6-dimethylpyridin-4-yl)phenyl]-7-methyl-8-(trifluoromethyl)-1,3-dihydro-1,5-benzodiazepin-2-one
RO5-4864
RO5-4864 (4’-Chlorodiazepam) is an atypical benzodiazepine with virtually no activity at the GABA-A receptor site.
Instead of reducing feelings of anxiety and muscle relaxation, RO5-4864 induces anxiety and causes muscles to contract. Despite these antithetical effects, RO5-4864 maintains a similar sedative action found in other benzodiazepine derivatives.
More research is needed to understand how this drug works — but it’s not likely to be of any interest to the designer drug community.
RO5-4864 Specs:
| Status: | Not Used |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 1688 |
| CAS#: | 14439-61-3 |
IUPAC Name: 7-Chloro-5-(2-chlorophenyl)-1-methyl-1,3-dihydro-2H1,4-benzodiazepin-2-one
RO07-5220
RO07-5220 (6′-Chlorodiclazepam) was under development by Hoffman-La Roche but was never completed. In preliminary testing, the drug showed characteristic anxiolytic and sedative qualities inherent to the benzodiazepine family.
It’s unclear why this drug wasn’t investigated further, but there are reports this compound has been detected on designer and street-level drug markets [112].
RO07-5220 Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 9975396 |
| CAS#: | 30144-88-8 |
IUPAC Name: 7-chloro-5-(2,6-dichlorophenyl)-1-methyl-3H-1,4-benzodiazepin-2-one
RO07-9749
RO07-9749 was never used in medicine but instead served as an internal standard for analyzing other benzodiazepines by its inventor, Hoffman-La Roche [113].
The safety and effect profile of this drug remains unclear.
RO07-9749 Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 3082318 |
| CAS#: | 30843-56-2 |
IUPAC Name: 5-(2-fluorophenyl)-7-iodo-1,3-dihydro-1,4-benzodiazepin-2-one
RO20-8065
RO20-8065 (8-Chloronorflurazepam) was primarily used for research purposes to help its developer, Hoffman-La Roche, better understand the benzodiazepine receptor subtypes on the GABA-A receptors [114].
It’s unclear why this compound wasn’t developed further or why Hoffman-La Roche chose this chemical, in particular, to help map out the receptors.
There are reports of this compound appearing as a designer drug, but this has not yet been confirmed.
RO20-8065 Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 10358803 |
| CAS#: | 88695-06-1 |
IUPAC Name: 7,8-dichloro-5-(2-fluorophenyl)-1,3-dihydro-1,4-benzodiazepin-2-one
SH-I-048A
SH-I-048A is most closely-related to flubromazepam and meclonazepam. It’s a non-subtype-selective superagonist at the BZD receptors [39]. This means it binds strongly to all subtypes of the BZD receptors.
A newer study from 2015 mapped the specific binding affinity for this drug on the BZD receptor subtypes [40]: α1 subtype — 0.77 nM; α2 subtype — 0.17 nM; α3 subtype — 0.38 nM; α5 subtype — 0.11 nM. No activity was reported at the α4 subtype.
As more research elucidates the distinct differences between each of these BZD subtypes, we can use information like this to determine unique effect profiles or use cases for benzodiazepine drugs. At the moment, it’s unclear what this non-specific binding capacity means compared to more selective drugs.
SH-I-048A Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 49850464 |
| CAS#: | 872874-11-8 |
IUPAC Name: (3S)-7-bromo-5-(2-fluorophenyl)-3-methyl-1,3-dihydro-1,4-benzodiazepin-2-one
Sulazepam
Sulazepam is a prodrug for three other active benzodiazepines — diazepam, desmethyldiazepam, and oxydiazepam.
This drug was studied in the 1970s in the Soviet Union but never made it to market. Preliminary testing showed traits characteristic of diazepam — anxiolytic, sedative-hypnotic, and muscle-relaxant [115].
Structurally, sulazepam is the thioamide derivative of diazepam.
This compound sometimes appears on designer drug markets but isn’t considered popular in any capacity. The specific effects of this substance are largely unknown.
Sulazepam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 17931 |
| CAS#: | 2898-13-7 |
IUPAC Name: 7-chloro-1-methyl-5-phenyl-3H-1,4-benzodiazepine-2-thione
Temazepam (Restoril)
Temazepam (Restoril) was first patented in 1962 and entered medical use in 1969. It was popular as a treatment for severe insomnia for many years but has since been replaced by various Z-drugs instead.
There are some reports this drug was used by the US military to help soldiers fall asleep during difficult or stressful situations.
This is an intermediate-acting benzodiazepine hypnotic with a half-life between 8 and 20 hours.
There are many generic versions of this drug available online, but none of them are particularly popular as designer drugs due to the high risk of uncomfortable side effects associated with its use (amnesia, grogginess, dizziness).
Temazepam Specs:
| Status: | Approved |
| Duration of Effects: | Intermediate-Acting |
| Common Dosage: | 15–30 mg |
| PubChem ID: | 5391 |
| CAS#: | 846-50-4 |
IUPAC Name: 7-chloro-3-hydroxy-1-methyl-5-phenyl-3H-1,4-benzodiazepin-2-one
Tetrazepam
Tetrazepam is sold under various brand names, including Clinoxan, Epsipam, Myolastan, Musaril, Relaxam, and Spasmorelax.
This compound has a potent anxiolytic, anticonvulsant, and antidepressant action — but much lower sedative or hypnotic qualities than other benzodiazepines.
Tetrazepam was used in Europe for several years but was eventually pulled after a report found the drug was associated with causing widespread immunological toxicity. This is a problem associated with many benzodiazepines but was especially predominant with this particular drug. This is the reason why one of the main side effects of long-term benzodiazepine use is frequent cold/flu and other infections.
Tetrazepam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Intermediate-Acting |
| Common Dosage: | 25–100 mg |
| PubChem ID: | 25215 |
| CAS#: | 10379-14-3 |
IUPAC Name: 7-chloro-5-(cyclohexen-1-yl)-1-methyl-3H-1,4-benzodiazepin-2-one
Tifluadom
Tifluadom is an atypical benzodiazepine with virtually no activity on the GABA-A receptors. This makes this drug entirely distinct from 90% of the benzodiazepine family.
Instead, this drug interacts with the κ-opioid receptor [41]. This mechanism is the target for a variety of psychotropic drugs, including ketamine and salvinorin-A (the active ingredient in Salvia divinorum).
This drug is not well studied but is believed to produce feelings of dysphoria, dissociation, and hallucinations. It’s sometimes used as a designer drug for this purpose, but there still isn’t much in the way of anecdotal reports of people who use this drug.
Tifluadom Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 115208 |
| CAS#: | 83386-35-0 |
IUPAC Name: N-[[5-(2-fluorophenyl)-1-methyl-2,3-dihydro-1,4-benzodiazepin-2-yl]methyl]thiophene-3-carboxamide
Tolufazepam
Tolufazepam is a designer benzodiazepine drug with known anxiolytic and muscle-relaxant qualities [52].
Not much information is available on this drug, with most of the research on this compound dating back to the 1980s. However, like many other benzos, interest in these novel compounds is picking up. Many of the older benzos that were discarded by the medical community are being dusted off and rebranded for the designer drug market — tolufazepam included.
Tolufazepam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 65647 |
| CAS#: | 86273-92-9 |
IUPAC Name: 7-chloro-5-(2-chlorophenyl)-1-[2-(4-methylphenyl)sulfonylethyl]-3H-1,4-benzodiazepin-2-one
Triflunordazepam
Triflunordazepam (AKA RO5-2904) is an obscure benzodiazepine derivative that’s been mentioned a few times in scientific studies examining the potency of fluorinated compounds.
One study, in particular, pointed out several molecules containing the CF3 group (including triflunordazepam) as being especially potent [53]. The examples given were trifluoperazine, flupentixol, aprepitant, isoflurane, sevoflurane, fluoxetine, fluvoxamine, celecoxib, fluramine, and a related benzodiazepine called quazepam.
The effects of this drug are not well known, and it has bever been tested for safety.
Triflunordazepam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 16795 |
| CAS#: | 2285-16-7 |
IUPAC Name: 5-phenyl-7-(trifluoromethyl)-1,3-dihydro-1,4-benzodiazepin-2-one
Uldazepam
Uldazepam was studied in Germany in the 1980s but never became an approved medication [116].
Not much is known about this substance, but there are a few reports this drug has been sold as a designer drug in places like France, Italy, and Spain.
Uldazepam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 34274 |
| CAS#: | 28546-58-9 |
IUPAC Name: 7-chloro-5-(2-chlorophenyl)-N-prop-2-enoxy-3H-1,4-benzodiazepin-2-amine
1,5-Benzodiazepines
The only difference between the 1,4-BZD class and the 1,5-BZD class is that the nitrogen in the central diazepam ring is in different locations [122]. The effects of the drugs in both classes are very similar, but some studies suggest subtle overall differences — such as the 1,5-BZDs having slightly less sedative action and are therefore better options for daytime use [117].
Most of the drugs in this class maintain the same classical benzodiazepine profile (anxiolytic, sedative-hypnotic, muscle-relaxant), but there are also a few with atypical effects (antifungal, anthelmintic [118], antiviral [119], analgesic, anti-inflammatory [120], and antipyretic [121]).
Arfendazam
Arfendazam is most similar to clobazam, but the effects are milder. This compound is only a partial agonist of the GABA-A receptors, so the hypnotic effects are reportedly much lower than other benzos on their own [54].
However, arfendazam is gradually metabolized into a separate benzodiazepine called lofendazam, which has much more characteristic hypnotic effects. Therefore, the effects of this drug gradually become more sedative over time.
Arfendazam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 65803 |
| CAS#: | 37669-57-1 |
IUPAC Name: Ethyl 7-chloro-4-oxo-5-phenyl-2,3-dihydro-1,5-benzodiazepine-1-carboxylate
Clobazam (Frisium & Onfi)
Clobazam is sold under the brand names Frisium, Onfi, and a long list of generics. It was first patented back in 1968 during the benzodiazepine boom and was marketed several years later as having fewer side effects than other benzodiazepine derivatives.
While this may be true, the incidence of rebound side effects such as anxiety, insomnia, and memory loss, remain high with this drug.
Clobazam is mildly popular in the designer drug community, with many proponents advocating the same idea that this drug somehow carries fewer risks of adverse effects.
Clobazam Specs:
| Status: | Approved |
| Duration of Effects: | Intermediate-Acting |
| Common Dosage: | 10–15 mg |
| PubChem ID: | 2789 |
| CAS#: | 22316-47-8 |
IUPAC Name: 7-chloro-1-methyl-5-phenyl-1,5-benzodiazepine-2,4-dione
CP-1414S
CP-1414S was patented in the 1970s and used as a less sedative benzo for managing muscle pain, anxiety, and epileptic seizures. It’s less used today in medicine but has been making a resurgence in the designer drug market.
The effects of this drug are most comparable to that of clobazam and share similar potency.
CP-1414S Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 37594 |
| CAS#: | 36985-40-7 |
IUPAC Name: 4-amino-8-nitro-1-phenyl-3H-1,5-benzodiazepin-2-one
Lofendazam
Lofendazam is closely related to clobazam, and one of its active metabolites, arfendazam. Not much is known about this drug in terms of safety and effects, and there are no pharmaceutical companies currently selling this medication.
This drug is somewhat popular as a designer drug, but due to the higher cost and closely similar effect profile to clobazam, the latter is more common.
Lofendazam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 71709 |
| CAS#: | 29176-29-2 |
IUPAC Name: 7-chloro-5-phenyl-2,3-dihydro-1H-1,5-benzodiazepin-4-one
Triflubazam
Triflubazam is similar to clobazam in terms of effects. It was studied in the 1970s as a potential anxiolytic and sedative but never made it to market.
This drug has a long half-life and duration of action [124], which makes it a poor candidate for insomnia treatment. Patients were waking up feeling groggy, and evidence suggested the drug reduced stage 3 sleep [125]. This means patients may stay asleep longer but wake feeling less rested.
Triflubazam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 31157 |
| CAS#: | 22365-40-8 |
IUPAC Name: 1-methyl-5-phenyl-7-(trifluoromethyl)-1,5-benzodiazepine-2,4-dione
2,3-Benzodiazepines
The 2,3-benzodiazepines (AKA 2,3-BZD analogs or GYKIs) are unlike conventional benzodiazepines in that they have little to no activity at the GABA-A receptor sites. Instead, this group primarily works through the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors [59], which are involved with rapid-fire synaptic transmission and neuroplasticity [60].
The primary applications of this group involve the treatment of neurodegenerative disorders such as Parkinson’s disease, Alzheimer’s disease (AD), Huntington’s chorea, and amyotrophic lateral sclerosis (ALS). A few are being explored as alternatives to amphetamines, and others are believed to be potential treatments for schizophrenia.
For the most part, you’re not going to find 2,3-BZD analogs on the designer drug market. Most are either far too obscure, don’t provide psychoactive effects, or are simply too costly to produce.
Girisopam
Girisopam (GYKI-51189, EGIS-5810) is most closely related to tofisopam and zometapine. Like other benzos, it’s anxiolytic but works through separate mechanisms from conventional benzodiazepines. This compound is interesting for its lack of sedative and muscle-relaxant qualities.
Unfortunately, there isn’t much official research on this compound as the drug never made it to market.
Girisopam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 71257 |
| CAS#: | 82230-53-3 |
IUPAC Name: 1-(3-chlorophenyl)-7,8-dimethoxy-4-methyl-5H-2,3-benzodiazepine
GYKI-52466
This atypical benzodiazepine doesn’t bind to GABA and subsequently doesn’t produce any of the characteristic hypnotic effects of other benzodiazepines. This compound acts as an AMPA receptor antagonist [55,56]. This effect gives GYKI-52466 muscle-relaxant qualities that are unlike that of conventional benzos.
There’s no indication this drug is being used on the designer drug market due to a lack of psychoactivity.
GYKI-52466 Specs:
| Status: | Not Used |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 3538 |
| CAS#: | 102771-26-6 |
IUPAC Name: 4-(8-methyl-9H-[1,3]dioxolo[4,5-h][2,3]benzodiazepin-5-yl)aniline
GYKI-52895
GYKI-52895 acts as a dopamine reuptake inhibitor (DARI), which is a unique mechanism for drugs in the benzodiazepine class. As a result, this compound has more in common with amphetamines and other stimulants than benzodiazepines and other hypnotics.
GYKI-52895 is the developmental name given to the drug by its inventor, Egis Pharmaceuticals. Usually, compounds are given names like this and then renamed when the drug becomes ready to undergo clinical trials. Clinical development for this drug began in 1997 as a treatment for Parkinson’s disease but was discontinued in 2001 before it could be given a proper name.
This drug remains obscure, but there’s a high likelihood it will start appearing in designer drug circles as an alternative to amphetamines.
The safety and dosage of this drug are unknown.
GYKI-52895 Specs:
| Status: | Not Used |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 3539 |
| CAS#: | 114460-08-1 |
IUPAC Name: 4-(8-methyl-8,9-dihydro-7H-[1,3]dioxolo[4,5-h][2,3]benzodiazepin-5-yl)aniline
Nerisopam
Nerisopam is chemically and pharmacologically similar to tofisopam (Emandaxin), which is used as an anxiety medication in Europe. Unlike other benzos, neither nerisopam nor tofisopam possesses strong sedative and muscle-relaxant qualities.
Nerosipam has very little research behind it. Only a few small animal trials have assessed the drug’s toxicity and mechanism of action [57]. These studies found that nerisopam primarily binds to the serotonin 5HT1 receptors and inhibits brain cAMP-phosphodiesterase. This is unlike the vast majority of benzodiazepines which work on the GABA-A receptors.
Nerisopam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 65875 |
| CAS#: | 102771-12-0 |
IUPAC Name: 4-(7,8-dimethoxy-4-methyl-5H-2,3-benzodiazepin-1-yl)aniline
Talampanel
Talampanel (GYKI 537773 and LY300164) is another atypical benzodiazepine working through the AMPA receptors rather than GABA receptors. It was explored for its potential use in treating ALS, malignant gliomas, and epilepsy but was abandoned after an unsatisfactory trial in 2010.
Psychoactive effects aren’t expected from this drug, so it’s unlikely to come across it in the designer drug space.
Talampanel Specs:
| Status: | Not Used |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 164509 |
| CAS#: | 161832-65-1 |
IUPAC Name: 1-[(8R)-5-(4-aminophenyl)-8-methyl-8,9-dihydro-[1,3]dioxolo[4,5-h][2,3]benzodiazepin-7-yl]ethanone
Tofisopam
Tofisopam is sold under the brand names Emandaxin, Grandaxin, and Sériel across Europe. It’s used as an anxiolytic and favored for its lack of sedative qualities [58]. Some studies suggest tofisopam attenuates the muscle-relaxant qualities of other benzos, muscimol, phenobarbitol, or other popular muscle-relaxant medications.
This drug is currently only approved in parts of Europe, but an isomer is currently being worked on by Vela Pharmaceuticals in New Jersey, USA.
This drug is sometimes found on designer drug markets. Despite being notably less hypnotic than other benzos, its long-term use results in the same extent of addiction as its more GABAergic derivatives.
Tofisopam Specs:
| Status: | Approved |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 5502 |
| CAS#: | 22345-47-7 |
IUPAC Name: 1-(3,4-dimethoxyphenyl)-5-ethyl-7,8-dimethoxy-4-methyl-5H-2,3-benzodiazepine
Triazolobenzodiazepines (TBZDs)
This group is home to several of the most common benzos used today — including alprazolam (Xanax), bromazolam (the most popular DBZD), and estazolam (ProSom). The majority of the other members of this group are either prodrugs, homologs, or metabolites of alprazolam.
What makes this group so popular is its intense potency. Many members in this group are among the strongest benzodiazepines currently available, including flunitrazolam, flunitrazepam, flubromazolam, triazolam, clonazolam, pynazolam, and flucotizolam.
The triazolobenzodiazepines are characterized by the presence of a triazole ring fused to a diazepine ring. The naming convention for this group is to use the suffix “-zolam.”
What makes this group special (beyond their increased potency) is their unique antidepressant qualities. These antidepressant effects sit on top of the classic hypnotic, anxiolytic, and muscle-relaxant effects inherent to the benzodiazepine family.
It’s believed this antidepressant action comes as a result of structural similarities between the triazolobenzodiazepines and tricyclic antidepressant medications.
Adinazolam
Adinazolam (Deracyn) was developed by Dr. Jackson Hester while working for Upjohn Company. Hester was seeking to enhance the antidepressant qualities of alprazolam (Xanax), which he is also credited for inventing.
The drug showed promise in early studies but never made it to market. The drug was associated with producing a higher degree of “mental unpleasantness” than other, more common benzodiazepines like diazepam or lorazepam.
The primary active metabolite of this drug is N-desmethyladinazolam [42].
Adinazolam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Short-Acting |
| Common Dosage: | Unspecified |
| PubChem ID: | 37632 |
| CAS#: | 37115-32-5 |
IUPAC Name: 1-(8-Chloro-6-phenyl-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepin-1-yl)-N,N-dimethylmethanamine
Alprazolam (Xanax)
As of 2019, alprazolam (Xanax) was one of the top 50 most prescribed medications in the United States.
This drug is mainly used for the short-term management of severe anxiety disorders and for the recreational ‘buzz’ it produces in higher doses. It’s short-acting and potent. The idea is that people can take it when they start to feel anxious to halt the episode in its tracks. The drug will then wear off a few hours later.
The short-lasting effects make it harder for the drug to accumulate in the body — which is a problem with some of the longer-lasting benzodiazepines.
Alprazolam was invented by Dr. Hester at the Upjohn Company (now part of Pfizer) and patented in 1971.
The patent for this medication expired decades ago so there are dozens of generic versions of this drug on the market today. Both Xanax and the drug’s many generic versions are widely available on designer drug markets as well as street-level vendors.
Some of the most common generic names for this drug include Alprax, Alprocontin, Alzam, Alzolam, Anzilum, Apo-Alpraz, Helex, Kalma, Mylan-Alprazolam, Niravam, Novo-Alprazol, Nu-Alpraz, Pacyl, Restyl, Tranax, Trika, Xycalm, Xanax, Xanor, Zolam, and Zopax
Alprazolam Specs:
| Status: | Approved |
| Duration of Effects: | Short-Acting |
| Common Dosage: | 0.5–1.5 mg |
| PubChem ID: | 2118 |
| CAS#: | 28981-97-7 |
IUPAC Name: 8-chloro-1-methyl-6-phenyl-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine
Balovaptan
Balovaptan (RG7314) is an atypical benzodiazepine that acts as a selective antagonist of the vasopressin V1A receptor (V1AR). This receptor plays a mysterious role in the human brain, and some researchers have referred to the gene encoding for this receptor as the “ruthlessness gene.” It’s believed to play a role in altruism (narcissistic versus empathetic tendencies) [126].
Most of the research involving this drug has been focused on its effects on managing autism spectrum disorder (ASD).
Recently, the FDA awarded balovaptan breakthrough therapy designation due to its ability to improve sociability in patients with ASD in preliminary clinical testing. However, the results of a Phase II study (published in March 2022) were disappointing. Researchers concluded that balovaptan did not improve social communication in autistic adults [43]. It’s not likely this drug will ever be taken to market.
Balovaptan Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 46200932 |
| CAS#: | 1228088-30-9 |
IUPAC Name: 8-chloro-5-methyl-1-(4-pyridin-2-yloxycyclohexyl)-4,6-dihydro-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine
Bromazolam
Bromazolam was first synthesized in 1976 but never marketed. It’s been revived as a recreational drug available on the grey market, where it was first reported in 2016. It’s sometimes sold under the development name XLI-268.
Bromazolam is the bromo version of alprazolam (it contains a chloride group instead).
Preliminary research suggests the binding capacity of bromazolam is weaker than alprazolam but maintains a higher affinity for the α1 BZD receptor subtype [40].
Bromazolam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 12562546 |
| CAS#: | 71368-80-4 |
IUPAC Name: 8-Bromo-1-methyl-6-phenyl-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine
Clonazolam
Clonazolam (AKA Clonitrazolam) is a hybrid between alprazolam (technically a triazolo- BZD) and clonazepam. The synthesis of this molecule was first reported in 1971 [45], but it was never approved for use.
This compound is used as a designer drug (first reported in 2015). It’s best known for having exceptional potency. High potency drugs are prevalent in the triazolobenzodiazepine class. However, the problem with this drug is that it carries a high risk for amnesic and sedative effects (prominent in doses as low as 0.5 mg).
Clonazolam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Intermediate-Acting |
| Common Dosage: | Unspecified |
| PubChem ID: | 12317881 |
| CAS#: | 33887-02-4 |
IUPAC Name: 6-(2-chlorophenyl)-1-methyl-8-nitro-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine
Estazolam (ProSom)
Estazolam (Prosom) was first patented in 1968 and eventually marketed as a medication for the management of insomnia in 1975.
Today, there are many generic forms of this drug, and it’s fairly popular as a research chemical. It’s a potent but short-acting hypnotic with clear intoxicating effects (half-life is 10–24 hours).
The major metabolite of estazolam is 4-hydroxyestazolam [46]. Others include 1-oxo-estazolam, 4′-hydroxy-estazolam, and benzophenone.
Estazolam Specs:
| Status: | Approved |
| Duration of Effects: | Intermediate-Acting |
| Common Dosage: | 1–2 mg |
| PubChem ID: | 3261 |
| CAS#: | 29975-16-4 |
IUPAC Name: 6-(2-Chlorophenyl)-1-methyl-8-nitro-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine
Fluadinazolam
Fluadinazolam was developed in 1973 but never marketed.
This compound is used as a designer drug, but not much is known about its specific effects. Based on its similarity to other TZBDs, it’s suspected this drug is highly potent.
Fluadinazolam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 21537253 |
| CAS#: | 28910-91-0 |
IUPAC Name: 1-[8-chloro-6-(2-fluorophenyl)-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepin-1-yl]-N,N-dimethylmethanamine
Flualprazolam
Flualprazolam is based on alprazolam but features an additional fluorine group. While there are plenty of exceptions, the addition of fluoride to functional groups on psychoactive molecules tends to increase their potency dramatically.
This drug was invented in 1976 but never made it to market. It’s only recently started appearing on the designer drug scene.
Flualprazolam can also be described as the triazole analog of both fludiazepam and flurazepam.
Flualprazolam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Intermediate-Acting |
| Common Dosage: | Unspecified |
| PubChem ID: | 10359044 |
| CAS#: | 28910-91-0 |
IUPAC Name: 8-Chloro-6-(2-fluorophenyl)-1-methyl-4Hbenzo[f][1,2,4]triazolo[4,3-a][1,4]diazepine
Flubromazolam
Flubromazolam (AKA liquid Xanax or JYI-73) is hands down one of the strongest benzodiazepines known. It’s extremely powerful, with sedative and amnesic actions present in doses as low as 0.5 mg [47] and fatal in doses over 3 mg [48].
For reference, the normal dose of diazepam is around 10 mg. Alprazolam, a drug that’s already associated with a higher risk of overdose [127], wasn’t found lethal until dosages exceeded 330 mg/kg (animal studies) (roughly 1000 times the normal human dose).
Flubromazolam was first reported in 2014 and has been gaining popularity in the designer drug community for its impressive potency ever since.
Flubromazolam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Intermediate-Acting |
| Common Dosage: | 0.2–0.4 mg |
| PubChem ID: | 21930924 |
| CAS#: | 612526-40-6 |
IUPAC Name: 8-Bromo-6-(2-fluorophenyl)-1-methyl-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine
Flunitrazolam
Flunitrazolam (AKA FNTZ or Flunazolam) was first reported in 2016. It’s the triazole analog of flunitrazepam (Rohypnol).
There was no mention of this substance in the scientific literature prior to its discovery by the EMCDDA Early Warning System in 2016.
The addition of the triazole ring for this TBZD significantly increases the potency of this drug, making it far more dangerous than Rohypnol — a common date-rape drug.
This drug is active at sub-milligram doses [49], which makes it particularly easy to overdose. The more potent the drug, the harder it is to accurately measure the dose and the less room for error.
Flunitrazolam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Short-Acting |
| Common Dosage: | 1–2 mg |
| PubChem ID: | 137700379 |
| CAS#: | 2243815-18-9 |
IUPAC Name: 6-(2-Fluorophenyl)-1-methyl-8-nitro-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine
Nitrazolam
Nitrazolam was first reported in 2015. It’s become one of the more popular benzodiazepines in niche circles for its distinct intoxicating properties.
This substance is closely related to clonazolam and flunitrazolam — only differing by the removal of a chlorine/fluorine group respectively at the benzene ring.
No safety data has been done on this drug, and the short and long-term side effects aren’t well defined.
Nitrazolam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 20317278 |
| CAS#: | 28910-99-8 |
IUPAC Name: 1-Methyl-8-nitro-6-phenyl-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine
Phenazolam
Phenazolam (AKA Clobromazolam, DM-II-90, and BRN 4550445) was invented in the 1980s but never made it into medical use.
This compound is the triazolo- version of another popular designer benzo, phenazepam. Reports suggest phenazolam is much more potent than phenazepam, especially regarding the hypnotic effects. However, specific dosages are not well defined.
Phenazolam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 1032830 |
| CAS#: | 87213-50-1 |
IUPAC Name: 8-bromo-6-(2-chlorophenyl)-1-methyl-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine
Pyrazolam
Pyrazolam (SH-I-04) was first reported in 2012 by a team led by Leo Sternbach at Hoffman-La Roche in the 1970s. Sternbach is credited as the first person to discover the benzodiazepine class.
This drug was studied for a while but never made it into medical use. It’s since been revived in the designer drug scene.
Pyrazolam shares similarities to alprazolam and bromazepam. Much like these other drugs, pyrazolam is notoriously potent.
What makes this drug unusual is that it isn’t metabolized by the body [47]. It’s excreted intact in the urine several hours after taking it.
Pyrazolam is most selective for the α2 and α3 subtypes of the GABAA receptor [51].
Pyrazolam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Intermediate-Acting |
| Common Dosage: | 0.75–1.5 mg |
| PubChem ID: | 12562545 |
| CAS#: | 39243-02-2 |
IUPAC Name: 8-Bromo-1-methyl-6-(pyridin-2-yl)-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine
Rilmazolam
Rilmazolam (CCRIS-1930) is an active metabolite of another drug, rilmazafone, which is an approved medication in Japan.
There isn’t much information available about rilmazolam, and it appears to be unpopular in the designer drug community. It’s hard to find, and there are virtually no reports available about its dosage or effect profile.
Rilmazolam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 148494 |
| CAS#: | 50330-59-1 |
IUPAC Name: 8-chloro-6-(2-chlorophenyl)-N,N-dimethyl-4H-[1,2,4]triazolo[1,5-a][1,4]benzodiazepine-2-carboxamide
Triazolam (Halcion)
Triazolam (Halcion) is a popular medication for treating severe insomnia. It was patented in the 1970s but didn’t go on sale until later in 1982.
This short-acting drug has a half-life of just 1.5 to 5 hours, and there are no active metabolites.
Like others in the triazolobenzodiazepine group, triazolam is exceptionally potent. Each standard Halcion pill is dosed with just 0.25 mg of the active ingredient.
There are many generic versions of triazolam on the market today and it’s often added as an adulterant to other pills to make them stronger.
Triazolam Specs:
| Status: | Approved |
| Duration of Effects: | Short-Acting |
| Common Dosage: | 0.25–0.5 mg |
| PubChem ID: | 5556 |
| CAS#: | 28911-01-5 |
IUPAC Name: 8-chloro-6-(2-chlorophenyl)-1-methyl-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine
Zapizolam
Zapizolam is currently the only member of the pyridotriazolodiazepine family, which is characterized by the combination of pyridine and triazole rings fused to a diazepine ring. There are other possible combinations that would fit in this group, but none of them have been researched formally. It’s unclear what the potential of this benzodiazepine subtype may have to offer.
Very little is known about zapizolam or where it was invented, but it’s been reported as a designer drug in several countries around Europe since 2015.
Clandestine chemists likely invented this compound in search of novel benzodiazepine compounds to sidestep various laws. The potency, safety, and effect profile are not well categorized, but the presence of a triazole ring indicates this compound could have potent effects similar to alprazolam (Xanax) or clonazolam (Klonopin).
Zapizolam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 68832 |
| CAS#: | 64098-32-4 |
IUPAC Name: 8-Chloro-6-(2-chlorophenyl)-4H-pyrido[2,3-f][1,2,4]triazolo[4,3-a][1,4]diazepine
Imidazobenzodiazepines
The imidazobenzodiazepine class contains a series of compounds that act as both agonists and inverse agonists to the GABA-A receptors [61].
Many of the agonistic drugs in this class are considered “partial agonist.” This is believed to make them far less prone to forming tolerance and dependency than most classical benzos. This means the members of this group are weaker but also far less likely to lead to significant dependency and withdrawal compared to other types of BZDs.
This class of benzos can be separated into three main groups:
- Classical benzodiazepine actions — drugs like bretazenil, EVT-201, loprazolam, and FG-8205 act in a similar capacity to conventional benzodiazepine drugs, acting as muscle relaxants, sedatives, anxiolytics, and intoxicants.
- Anaesthetic agents — drugs like midazolam, climazolam, and remimazolam are powerful sedatives and amnesic agents for use during surgical procedures.
- Benzodiazepine Antidotes — flumazenil, imidazenil, and sarmazenil competitively block and bind to the BZD receptors. They’re useful for treating acute benzodiazepine overdose.
- Novel Nootropic Substances — a selection of imidazobenzodiazepines (FG-8094, GL-II-73, PWZ-029, and RO4938581) act as inverse agonists with high specificity to the α5 subtype of the GABA-A receptors. This action is theorized to enhance one’s capacity for learning and memory.
This drug is of interest to pharmacologists looking to design new social or recreational drugs. The most promising candidate, bretazenil, has all the characteristics of social GABAergics like alcohol or GHB but carries far less risk. Of course, a substantial risk is still omnipresent among all members of this family. It’s very likely we’ll see more interest in this BZD subgroup over the next few decades.
Bretazenil
Bretazenil is an anxiolytic benzodiazepine revered for its reduced capacity for causing dependency and withdrawal despite maintaining strong GABAergic activity [62]. Despite its promising effects and a higher level of safety, bretazenil and several related analogs were never approved as medications. They’ve only recently entered the focus of drug developers — clandestine and pharmaceutical alike.
This compound is most similar (structurally) to flumazenil, but the effects are distinct.
Infamous psychopharmacologist and researcher David Nutt, once suggested bretazenil as a potential jumping-off point for developing an ideal social drug. The effects of this compound are similar to alcohol intoxication without the downsides of aggression or nausea. It also appears to lack liver-toxic effects, and drugs exist that can quickly reverse the effects of overdosage (flumazenil).
Bretazenil Specs:
| Status: | Research Chemical |
| Duration of Effects: | Short-Acting |
| Common Dosage: | Unspecified |
| PubChem ID: | 107926 |
| CAS#: | 84379-13-5 |
IUPAC Name: ert-butyl (7S)-14-bromo-12-oxo-2,4,11-triazatetracyclo[11.4.0.02,6.07,11]heptadeca-1(17),3,5,13,15-pentaene-5-carboxylate
Climazolam (Climasol)
Climazolam is sold under the trade name Climasol by the Swiss Pharmaceutical company Gräub. It’s a veterinary medication used for the anesthesia of dogs, cats, and horses.
This compound is highly sedative and, therefore, very dangerous. It’s relatively popular in parts of Europe due to the fact that it’s relatively easy to get this medication on the black market. Most of it comes from products stolen from veterinary clinics.
Climazolam Specs:
| Status: | Approved (Veterinary Use) |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 68790 |
| CAS#: | 59467-77-5 |
IUPAC Name: 8-chloro-6-(2-chlorophenyl)-1-methyl-4H-imidazo[1,5-a][1,4]benzodiazepine
EVT-201
EVT-201 was developed by Roche as a non-sedating anxiolytic. However, the phase I clinical trials showed marked hypnotic effects in patients, so it was discontinued and sold to Evotec. It’s now entering the second round of phase III clinical testing as a treatment for insomnia, where it’s believed to be a safer option for seniors than other benzodiazepine medications.
It’s rare to find this medication on the grey market, and the drug is currently patented by Evotec.
EVT-201 Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 9885841 |
| CAS#: | 308239-86-3 |
IUPAC Name: 7-chloro-3-[5-[(dimethylamino)methyl]-1,2,4-oxadiazol-3-yl]-5-methyl-4H-imidazo[1,5-a][1,4]benzodiazepin-6-one
FG-8094
FG-8094 (AKA L-655,708) is a synthetic nootropic invented in 1996 by Merck & Co.
This compound targets the same GABA-A receptors as most other benzodiazepines, but it has unique properties from other benzodiazepines in how it binds to these receptors. FG-8094 acts as an inverse agonist at the BZD binding sites, with a particularly strong affinity for the α5 subtype [70].
There’s some evidence that the α5 BZD receptor is primarily involved with the amnesic effects of benzodiazepines (and other amnesic drugs) [71]. This receptor is prevalent in the hippocampus region of the brain, which is intimately involved in the formation and retrieval of memories.
Based on this information, it’s possible that FG-8094 or other inverse α5 agonists could have a positive impact on memory and learning.
Interestingly, animal studies have found ketamine-like antidepressant effects from a related inverse α5 antagonist, MRK-016 [72]. More research is needed to better understand the extent of this novel nootropic mechanism.
FG-8094 Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 5311203 |
| CAS#: | 130477-52-0 |
IUPAC Name: ethyl (7S)-15-methoxy-12-oxo-2,4,11-triazatetracyclo[11.4.0.02,6.07,11]heptadeca-1(17),3,5,13,15-pentaene-5-carboxylate
FG-8205
FG-8205 (L-663,581) is most closely related to bretazenil, which is believed to be one of the “safer” benzodiazepines available. Not much research is available on FG-8205, but preliminary reports suggest it maintains similar potency to bretazenil, but with less tendency towards sedation.
There’s a good chance this drug will start appearing on the designer drug market in the near future. If it’s true that this substance maintains similar effects to bretazenil — which is already a popular social drug — but with less of the sedation that makes these drugs undesirable, this could become one of the go-to choices for people who like the effects of benzos.
FG-8205 Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 129710 |
| CAS#: | 122384-14-9 |
IUPAC Name: 7-chloro-5-methyl-3-(5-propan-2-yl-1,2,4-oxadiazol-3-yl)-4H-imidazo[1,5-a][1,4]benzodiazepin-6-one
Flumazenil (Anexate)
Flumazenil (AKA flumazepil) is an antidote for benzodiazepine overdose. It acts as a competitive inhibitor to conventional benzodiazepine medications. It’s also used to reverse anesthesia brought on by hypnotic benzodiazepines like flunitrazepam.
This compound is available in both injectable and intranasal forms. The goal of using this medication is to get it into the bloodstream as quickly as possible to counteract an overdose.
Flumazenil has been on the market since 1991 but was invented ten years earlier in a Hoffman-La Roche laboratory.
Flumazenil Specs:
| Status: | Approved |
| Duration of Effects: | Short-Acting |
| Common Dosage: | Unspecified |
| PubChem ID: | 3373 |
| CAS#: | 78755-81-4 |
IUPAC Name: ethyl 8-fluoro-5-methyl-6-oxo-4H-imidazo[1,5-a][1,4]benzodiazepine-3-carboxylate
GL-II-73
GL-II-73 is sometimes sold as a nootropic research chemical. Not much testing has been done on this substance, but many users claim it improves cognitive performance when used in small doses.
Preliminary research has shown that this compound primarily targets the α5 BZD binding site on the GABA-A receptor and has no affinity whatsoever for the α1 site.
More research is needed to understand if and why GL-II-73 has nootropic benefits and whether or not it’s safe to use as such.
GL-II-73 Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 130439778 |
| CAS#: | Not Listed |
IUPAC Name: (4R)-8-ethynyl-6-(2-fluorophenyl)-N,N,4-trimethyl-4H-imidazo[1,5-a][1,4]benzodiazepine-3-carboxamide
I-Iomazenil
I-Iomazenil (AKA benzodine) was developed by Hoffmann-La Roche to help them research benzodiazepine receptors in the early 1990s. The iodine portion of the molecule can be picked up by special lab equipment to help researchers identify how and where the molecule is binding to the receptor.
Today, this compound is being explored as a potential treatment for alcohol withdrawal disorder. It acts as a partial inverse agonist and antagonist of the BZD receptors.
This compound is structurally related to flumazenil, which is another BZD antagonist used as an antidote for withdrawal symptoms.
There is little value for this drug on the designer drug market as it has little, if any, intoxicating effects. In fact, this drug is suggested to offer a potential antidote for drunkenness by binding to the receptors and blocking the effects of alcohol.
I-Iomazenil Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 65959 |
| CAS#: | 127396-36-5 |
IUPAC Name: ethyl 7-(123I)iodanyl-5-methyl-6-oxo-4H-imidazo[1,5-a][1,4]benzodiazepine-3-carboxylate
Imidazenil
Imidazenil is a unique benzo in that it appears to inhibit the sedative and amnesic effects of benzodiazepines such as diazepam but has no effect on the seizure threshold [69]. This is significant because current antidotes, such as flumazenil can increase the severity of withdrawal symptoms and reduce the seizure threshold — thus increasing the chance of seizures during the withdrawal phase.
Imidazenil could see use as an alternative to current benzodiazepine antidotes and may even prove useful in its own right as a non-sedative anxiolytic.
The intoxicating profile of this benzo is not well understood, and it doesn’t appear this drug is available on designer drug markets at the moment.
Imidazenil Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 119194 |
| CAS#: | 151271-08-8 |
IUPAC Name: 6-(2-bromophenyl)-8-fluoro-4H-imidazo[1,5-a][1,4]benzodiazepine-3-carboxamide
Loprazolam (Triazulenone)
Loprazolam (Triazulenone and Dormonoct) has been in medical use since 1983. The patents have long since expired, and there are now dozens of manufacturers making generic forms of this relatively popular insomnia medication.
The dose of this drug is very low at just 1 mg. This makes loprazolam roughly ten times stronger than diazepam (Valium).
Like many potent benzodiazepines, this compound can be found on the designer drug market. It’s usually taken alongside stimulants to reduce the sedative qualities and allow users to enter a state of intoxication comparable to GHB. This is a very dangerous way of using these compounds.
Loprazolam Specs:
| Status: | Approved |
| Duration of Effects: | Intermediate-Acting |
| Common Dosage: | 0.5–1.5 mg |
| PubChem ID: | 3033860 |
| CAS#: | 61197-73-7 |
IUPAC Name: (2Z)-6-(2-chlorophenyl)-2-[(4-methylpiperazin-1-yl)methylidene]-8-nitro-4H-imidazo[1,2-a][1,4]benzodiazepin-1-one
Midazolam (Versed)
Midazolam (Versed) is mainly used as an anesthetic and treatment for severe insomnia. It’s also powerfully amnesic — directly blocking the ability to form new memories while the drug remains in effect.
Unlike dissociative anesthetics like xenon gas or nitrous oxide, midazolam (and most benzos) don’t actually cause the patient to become unconscious, though the intense level of sedation makes it appear that they are.
This drug is listed on the World Health Organizations List of Essential Medicines but is also tightly controlled. This drug is extremely dangerous and should never be used for anything outside medical practice.
Many of the side effects of using this drug go unnoticed because of the problems with memory it causes. It’s common for people to have no recollection of even taking the drug several hours after it’s worn off.
Midazolam Specs:
| Status: | Approved |
| Duration of Effects: | Short-Acting |
| Common Dosage: | 10–15 mg |
| PubChem ID: | 4192 |
| CAS#: | 59467-70-8 |
IUPAC Name: 8-chloro-6-(2-fluorophenyl)-1-methyl-4H-imidazo[1,5-a][1,4]benzodiazepine
PWZ-029
PWZ-029 is a novel nootropic substance currently under development by WiSys.
It’s reported to act as an inverse α5 agonist with a minor action as a partial inverse α3 agonist. This action on the α5 BZD receptor subtypes is similar to that of FG-8094 and MRK-016 — both of which have shown similar potential as a nootropic.
The nootropic effects of this drug are reported to involve improved capacity for (passive) learning and memory without sedative or anxiety-inducing side effects.
However, at least one study involving this drug suggests sedative action in doses higher than 10 mg/kg [73].
PWZ-029 Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 9971547 |
| CAS#: | 164025-33-6 |
IUPAC Name: 8-chloro-3-(methoxymethyl)-5-methyl-4H-imidazo[1,5-a][1,4]benzodiazepin-6-one
Remimazolam (Byfavo)
Remimazolam (Byfavo) is used as a shorter-acting alternative to midazolam as a sedative. This drug is generally reserved for shorter procedures (30 minutes or less). It has a much faster onset and wears off more quickly than midazolam [74]. Patients also recover more quickly and experience less risk of side effects when using this drug. However, its effects are too short to be of use for more substantial surgical procedures.
This drug was only approved recently in 2020 in the United States and 2021 in the European Union.
There’s no indication this drug is being used on the designer drug market at the moment. Its powerful sedative and amnesic qualities make it a poor choice for recreational use in any capacity.
Remimazolam Specs:
| Status: | Approved |
| Duration of Effects: | Short-Acting |
| Common Dosage: | Unspecified |
| PubChem ID: | 9867812 |
| CAS#: | 308242-62-8 |
IUPAC Name: methyl 3-[(4S)-8-bromo-1-methyl-6-pyridin-2-yl-4H-imidazo[1,2-a][1,4]benzodiazepin-4-yl]propanoate
RO48-6791
Ro48-6791 was first developed in the 1990s by Hoffman-La Roche but later abandoned.
This drug acts similarly to midazolam as a fast-acting anesthetic. Modifications in the chemical structure allow this drug to dissolve in water, which has been found to increase the bioavailability and increase the potency of the drug by nearly 4X compared to midazolam [75].
This compound was abandoned due to a lack of clear improvement (besides higher potency) and substantially increased risk for side effects (primarily extreme dizziness).
A series of related compounds were explored by Roche in the 90s, including RO48-6791 and a similar compound called RO48-8684. Neither of these drugs ever made it to market.
RO48-6791 Specs:
| Status: | Not Used |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 9953485 |
| CAS#: | 172407-17-9 |
IUPAC Name: 3-[5-[(dipropylamino)methyl]-1,2,4-oxadiazol-3-yl]-8-fluoro-5-methyl-4H-imidazo[1,5-a][1,4]benzodiazepin-6-one
RO4938581
This research compound was first reported by Hoffman-La Roche in 2009. It’s one of several benzodiazepines in the imidazobenzodiazepine subgroup thought to provide nootropic benefits. This compound acts as an inverse agonist on the α5 BZD receptor subtype [76]. This mechanism is believed to improve learning and memory.
This compound, along with another functional analog, basmisanil (RO5186582), has been explored as a potential treatment for down syndrome [77].
RO4938581 Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 11624499 |
| CAS#: | 883093-10-5 |
IUPAC Name: 3-Bromo-10-(difluoromethyl)-9H-benzo[f]imidazo[1,5-a][1,2,4]triazolo[1,5-d][1,4]diazepine
Sarmazenil
Sarmazenil (RO15-3505) was developed by Hoffman-La Roche in the hopes of discovering a new cognitive-enhancement medication. It’s classified as an inverse agonist to the BZD receptors [78]. This means it works directly opposite to most classical benzodiazepines but lacks the specificity to the α5 subtype that gives some benzos nootropic benefits.
Sarmazenil is mainly reserved for veterinary use to reawaken anesthetized animals more quickly.
This drug is not popular in the designer drug world, nor is it likely to become popular. This drug actively induces feelings of anxiety and muscle tension. It also has a high affinity for causing seizures.
Sarmazenil Specs:
| Status: | Approved (Veterinary Medicine) |
| Duration of Effects: | Intermediate-Acting |
| Common Dosage: | Unspecified |
| PubChem ID: | 71231 |
| CAS#: | 78771-13-8 |
IUPAC Name: Ethyl 7-chloro-5-methyl-6-oxo-5,6-dihydro-4H-imidazo[1,5-a][1,4]benzodiazepine-3-carboxylate
SH-053-R-CH3-2′F
SH-053-R-CH3-2′F is a research chemical with a particularly high affinity for the α5 subtype of the GABA-A receptors. Benzodiazepines that have an inverse action (inhibitory effect) on these receptors are thought to improve memory and learning. This drug binds and activates these receptors, so it’s used as a means of testing the binding affinity for new α5 inhibitors.
Because of the role of α5 in the amnesic qualities of other drugs, it’s thought the high specificity of this drug for these receptors could make it a particularly strong amnesic agent.
SH-053-R-CH3-2′F Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 11574585 |
| CAS#: | 872874-14-1 |
IUPAC Name: (R)-8-ethynyl-6-(2-fluorophenyl)-4-methyl-4H-2,5,10b-triaza-benzo[e]azulene-3-carboxylic acid ethyl ester
Oxazolobenzodiazepines
This relatively small group of benzodiazepines is home to a number of long-lasting chemicals that act as prodrugs for other benzodiazepines.
None of the members in this group are very popular in medicine, but a few have earned their place in the designer drug market due to the impressive duration of effects.
Drugs like flutazolam, for example, kick in with very strong but short-lasting inebriating effects that transition into more classical anxiolytic and hypnotic action as it metabolizes into the long-lasting norflurazepam (duration of effects of this metabolite is over 10 hours in total).
Cloxazolam (Akton)
Cloxazolam (Akton, Cloxam, Clozal, Elum, Olcadil, and Sepazon) is a rather obscure and rarely used benzodiazepine medication. It’s available in Argentina, Australia, Portugal, Switzerland, Luxembourg, Germany, Taiwan, and Japan as a prescription anxiety medication.
The effects of cloxazolam are reportedly similar to bromazepam, with roughly 3 times the relative potency [79].
Cloxazolam made an entrance into the designer drug market only very recently but is not a popular option.
Cloxazolam Specs:
| Status: | Approved |
| Duration of Effects: | Long-Acting |
| Common Dosage: | Unspecified |
| PubChem ID: | 2816 |
| CAS#: | 24166-13-0 |
IUPAC Name: 10-chloro-11b-(2-chlorophenyl)-2,3,5,7-tetrahydro-[1,3]oxazolo[3,2-d][1,4]benzodiazepin-6-one
Flutazolam (Coreminal)
Flutazolam (Coreminal) is primarily used in Japan as a more sedative alternative to diazepam. The potency of these two medications is almost identical. Flutazolam is also reported to have a more inebriating effect than diazepam and is therefore preferred by people seeking the recreational effects.
The half-life of flutazolam is very short — just 3–5 hours. But its primary active ingredient, n-desalkylflurazepam (norflurazepam) is substantially longer-lasting — roughly 50–100 hours in total.
Flutazolam Specs:
| Status: | Approved |
| Duration of Effects: | Long-Acting |
| Common Dosage: | Unspecified |
| PubChem ID: | 3398 |
| CAS#: | 27060-91-9 |
IUPAC Name: 10-chloro-11b-(2-fluorophenyl)-7-(2-hydroxyethyl)-3,5-dihydro-2H-[1,3]oxazolo[3,2-d][1,4]benzodiazepin-6-one
Haloxazolam (Somelin)
Haloxazolam (Somelin) is primarily sedative, with similar qualities to triazolam, temazepam, and flunitrazepam.
Interestingly, this substance has been found to affect the gamma motor neurons [80]. Most benzos that bind to these neurons target both the alpha and gamma motor neurons. The alpha motor neurons are responsible for controlling muscle contraction, while the gamma neurons are associated with stretch and flex limits [81].
Haloxazolam Specs:
| Status: | Approved |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 3563 |
| CAS#: | 59128-97-1 |
IUPAC Name: 10-bromo-11b-(2-fluorophenyl)-2,3,5,7-tetrahydro-[1,3]oxazolo[3,2-d][1,4]benzodiazepin-6-one
Mexazolam (Melex)
Mexazolam (Melex and Sedoxil) is an approved anxiety medication in Portugal. It has been detected in street-level benzodiazepines but is very rare.
There are two active metabolites of this compound — delorazepam and lorazepam. Both of these metabolites are also approved medications (Dadumir and Ativan, respectively).
Mexazolam Specs:
| Status: | Approved |
| Duration of Effects: | Long-Acting |
| Common Dosage: | 0.25–2 mg |
| PubChem ID: | 4177 |
| CAS#: | 31868-18-5 |
IUPAC Name: 10-chloro-11b-(2-chlorophenyl)-3-methyl-2,3,5,7-tetrahydro-[1,3]oxazolo[3,2-d][1,4]benzodiazepin-6-one
Oxazolam (Nordaz)
Oxazolam is a prodrug for nordiazepam (desmethyldiazepam) [82], which is an FDA-approved medication sold under brand names like Nordaz and Stilny. Nordiazepam is considered one of the longest-lasting benzos on the market today. There are many other drugs or substances that serve as prodrugs for nordazepam, including prazepam and clorazepate.
This substance was discovered in the 70s and tested throughout the 80s to determine its effects but was never completed. There are some indications this substance is being sold on the grey market as a “legal” alternative to conventional benzos. It shares a virtually identical effect profile to nordiazepam.
Oxazolam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Short-Acting |
| Common Dosage: | 10–25 mg |
| PubChem ID: | 62779 |
| CAS#: | 27167-30-2 |
IUPAC Name: (2R,11bS)-10-chloro-2-methyl-11b-phenyl-2,3,5,7-tetrahydro-[1,3]oxazolo[3,2-d][1,4]benzodiazepin-6-one
Thienodiazepines
This small class of benzodiazepines contains just bentazepam, clotiazepam, and the research chemical RO09-9212. They consist of a diazepine ring fused to a thiophene ring.
The subgroup thienotriazolodiazepines contain an additional triazole ring to this chemical structure which makes them particularly potent. Some of the strongest benzodiazepines currently known sit in this group, including flubrotizolam and fluclotizolam. The triazole ring is found in numerous high-potency benzodiazepines, including alprazolam, flubromazolam, and flunitrazolam.
The effects of both groups are very similar to that of the more common 1,4-benzodiazepines — which include compounds such as diazepam (Valium) as well as some of the more popular triazolobenzodiazepines such as alprazolam (Xanax).
Bentazepam
Bentazepam (AKA Thiadipone and Tiadipona) is a simple thienodiazepine with short-acting (2–4 hour) effects. The elimination half-life (the time it takes to remove exactly half of the drug from the bloodstream) is estimated to be nearly 3 hours quicker than conventional short-acting benzos [83].
The duration of effects of this drug is short — just 2–4 hours — so the risk of dependency is higher than that of many other benzodiazepines. Shorter effects encourage more redosing, which strongly increases habit formation.
Bentazepam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Short-Acting |
| Common Dosage: | Unspecified |
| PubChem ID: | 135412795 |
| CAS#: | 29462-18-8 |
IUPAC Name: 5-phenyl-3,4,6,7,8,9-hexahydro-[1]benzothiolo[2,3-e][1,4]diazepin-2-one
Brotizolam (Lendormin)
Brotizolam (Lendormin) is a thienotriazolodiazepine compound with potent sedative-hypnotic effects similar to other popular drugs like triazolam or midazolam.
As a short-acting hypnotic and anxiolytic, Lendormin is primarily used for treating acute anxiety and insomnia in Europe and Southeast Asia (not approved in the US, Canada, or UK).
This drug is exceptionally potent — the active dose is just 0.125 to 0.25 milligrams [84]. Brotizolam is an intermediate-acting benzodiazepine at around 5 hours (though this is on the short-end of intermediate).
Brotizolam is a less common choice for recreational drug users due to its strong sedative action, but because of its impressive potency, it’s been found as an adulterant in a lot of street-level benzos.
Brotizolam Specs:
| Status: | Approved |
| Duration of Effects: | Short-Acting |
| Common Dosage: | 0.2–0.4 mg |
| PubChem ID: | 2451 |
| CAS#: | 57801-81-7 |
IUPAC Name: 2-Bromo-4-(2-chlorophenyl)-9-methyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepine
Ciclotizolam
As one of the weaker members of the thienotriazolodiazepine class, ciclotizolam (WE-973) never made it through early development. There were too many other, much stronger derivatives in this or related classes to go after instead.
Ciclotizolam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 71949 |
| CAS#: | 58765-21-2 |
IUPAC Name: 8-bromo-6-(o-chlorophenyl)-1-cyclohexyl-4H-5-triazolo(3,4-c)thieno(2,4-e)-1,4-diazepine
Clotiazepam (Clozan)
Clotiazepam (sold as Clozan, Distensan, Rize, and Veratran) is a simple thienodiazepine with powerful sedative action. In lower doses, it’s used as a treatment for anxiety and for its muscle-relaxant effects.
What makes coltiazepam different is its ability to increase stage 2 non-rem (NREM) sleep without affecting the amount of time in REM [85]. This is significant because many benzodiazepine sleeping pills increase the amount of time asleep but have a negative impact on the most important stages of sleep, including both stage 2 NREM (deep sleep) and REM.
Clotiazepam Specs:
| Status: | Approved |
| Duration of Effects: | Short-Acting |
| Common Dosage: | 5–10 mg |
| PubChem ID: | 2811 |
| CAS#: | 33671-46-4 |
IUPAC Name: 5-(2-chlorophenyl)-7-ethyl-1-methyl-3H-thieno[2,3-e][1,4]diazepin-2-one
Deschloroetizolam
Deschloreotizolam was first reported on the grey/black markets in 2014 and continues to be one of the more popular substances in certain areas. It’s classified as a thienotriazolodiazepine, so the effects are subjectively very similar to Valium or other conventional 1,4-benzodiazepines.
This compound is sometimes sold under the names Etizolam-2, Etiz-dechlorinate, or Legal-Eti on designer drug markets.
Deschloroetizolam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 827322 |
| CAS#: | 40054-73-7 |
IUPAC Name: 4-Phenyl-2-ethyl-9-methyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepine
Etizolam (Depas)
Etizolam (Depas) was originally developed in Japan and brought to market in 1984.
The patents for this medication have long expired, so there are dozens of generic versions of this drug available today. Some of the names this drug is sold under include Arophalm, Capsafe, Depas, Dezolam, Eticalm, Etidrale, Etisedan, Etizolam, Guperies, Medipeace, Mozun, Nonnerv, Palgin, Pasaden, Sedekopan, and Sylazepam.
This substance is nearly six times as potent as diazepam yet remained unregulated until 2019.
Etizolam managed to escape blanket regulations on benzodiazepines because its chemical composition was distinct from known substances at the time — including alprazolam (Xanax) and diazepam (Valium). Today etizolam is classified as a Schedule IV substance along with the vast majority of other benzodiazepine derivatives.
Early drug detection agencies in the US first reported this substance in 2011, and has since become one of the most popular “recreational” benzodiazepines on the designer drug market (along with flualprazolam). Etizolam is sold under street names like Etiz, Etizzy, and Etizest.
This compound is cheap to buy and has a strong intoxicating effect profile on top of the usual anxiolytic, sedative-hypnotic, and muscle-relaxant qualities.
Chemically, etizolam is classified as a thienotriazolodiazepine, which places it somewhere in the middle between Valium (diazepam) and Xanax (alprazolam) in terms of chemical structure.
Etizolam Specs:
| Status: | Approved |
| Duration of Effects: | Short-Acting |
| Common Dosage: | 1–2 mg |
| PubChem ID: | 3307 |
| CAS#: | 40054-69-1 |
IUPAC Name: 4-(2-Chlorophenyl)-2-ethyl-9-methyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepine
Flubrotizolam
Flubrotizolam is predicted to be one of the strongest NBZD substances currently available (along with clonazolam, pynazolam, and flucotizolam [86]. It’s potent at sub-milligram doses and, therefore, is a common adulterant in other, more conventional benzodiazepine drugs produced in clandestine labs.
Flubrotizolam and flucotizolam are both classified as thienotriazolodiazepines, while pynazolam is a member of the closely-related triazolobenzodiazepine group.
Flubrotizolam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 3044878 |
| CAS#: | 57801-95-3 |
IUPAC Name: 4-bromo-7-(2-fluorophenyl)-13-methyl-3-thia-1,8,11,12-tetrazatricyclo[8.3.0.02,6]trideca-2(6),4,7,10,12-pentaene
Fluclotizolam
Fluclotizolam was first invented in the late 70s but never made it to market. Today it’s primarily sold as a powerful hypnotic designer drug.
This compound is most closely related to other thienotriazolodiazepine derivatives such as flubrotizolam. The only difference is that fluclotizolam has a chlorine group, whereas flubrotizolam contains bromine. Both drugs are powerful and relatively short-lasting (around 3 hours).
Fluclotizolam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 21317700 |
| CAS#: | 54123-15-8 |
IUPAC Name: 2-Chloro-4-(2-fluorophenyl)-9-methyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a]diazepine
Fluetizolam
Fluetizolam was patented in Japan by Yoshitomi Pharmaceutical in the 90s but never made it to market. It’s been “rediscovered” as a designer drug and is revered for being a particularly potent alternative to etizolam. The threshold dose for this substance is well below 1 mg.
Fluetizolam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 12434320 |
| CAS#: | 40054-88-4 |
IUPAC Name: 4-ethyl-7-(2-fluorophenyl)-13-methyl-3-thia-1,8,11,12-tetrazatricyclo[8.3.0.02,6]trideca-2(6),4,7,10,12-pentaene
Israpafant
Israpafant (Y-24180) is classified as a thienotriazolodiazepine with a similar chemical structure to etizolam. However, the effects of this drug are entirely distinct from other members of the group.
This compound only weakly binds to the GABA-A receptors and instead attaches itself to the platelet-activating factor receptor, where it acts as a selective antagonist (inhibitor) [87]. It’s also been found to inhibit the activation of eosinophil cells [88]. Most of the interest around this drug is in the development of new immunological or antiasthma therapies.
There are no psychoactive effects expected from this atypical benzodiazepine.
Israpafant Specs:
| Status: | Not Used |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 636426 |
| CAS#: | 136624-40-3 |
IUPAC Name: (6R)-4-(2-Chlorophenyl)-2-[2-(4-isobutylphenyl)ethyl]-6,9-dimethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepine
Metizolam
Metizolam was first reported as a new designer drug in 2015 by the early warning detection system in the EU. It’s one of the active metabolites of etizolam and shares similar potency and effects but a shorter duration of action.
Metizolam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Short-Acting |
| Common Dosage: | Unspecified |
| PubChem ID: | 12434325 |
| CAS#: | 40054-68-0 |
IUPAC Name: 4-(2-Chlorophenyl)-2-ethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepine
Thienobenzodiazepines
The thienobenzodiazepines are a small group of benzodiazepine derivatives characterized by the fusion of a diazepine ring with a thiophene ring and a benzene ring.
This group has been reported to be selective for the α2 subunit of the GABA-A receptor.
This group is considered atypical in that it doesn’t produce the effects characteristic of most benzodiazepine drugs. There’s little, if any, interest in the thienobenzodiazepine group for people interested in psychoactive effects.
Olanzapine (Zyprexa)
Olanzapine (Zyprexa) was first patented in 1991 and later approved for sale in 1996. The patent has since expired, and there are now numerous generic versions, as well as a brand-name drug sold by Eli Lilly that combines both olanzapine and fluoxetine (Symbyax).
The effects of this drug are completely different from other benzodiazepines. Instead of targeting the GABA-A receptors to form a hypnotic and anxiolytic effect, this drug targets the serotonin (5HT2A and 5HT1A) receptors, dopamine (D2) receptors [89], P-glycoprotein [90], and muscarinic M3 receptors [91]. It’s used as a treatment for psychotic disorders such as schizophrenia and bipolar disorder.
Olanzapine is not used as a recreational drug and does not have desirable psychoactive effects.
Olanzapine Specs:
| Status: | Approved |
| Duration of Effects: | Long-Acting |
| Common Dosage: | 5–10 mg |
| PubChem ID: | 135398745 |
| CAS#: | 132539-06-1 |
IUPAC Name: 2-Methyl-4-(4-methyl-1-piperazinyl)-10H-thieno[2,3-b][1,5]benzodiazepine
Pyridodiazepines
There are very few drugs in this unique class of benzodiazepine derivatives. The most relevant is lopirazepam, which maintains the characteristic hypnotic, anxiolytic, and muscle-relaxant qualities inherent to the benzodiazepine family.
Lopirazepam
Lopirazepam was invented in the 1970s but never made it to market. At the time, one of the biggest hurdles companies were trying to solve with new benzodiazepines was the negative impact these drugs had on REM sleep. In at least one study, lopirazepam was found to shorten REM sleep cycles [92].
Not much is known about this drug, and interest as a designer drug has only grown slightly in recent years.
Lopirazepam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 68672 |
| CAS#: | 42863-81-0 |
IUPAC Name: (RS)-7-Chloro-5-(2-chlorophenyl)-3-hydroxy-1,3-dihydro-2H-pyrido[3,2-e][1,4]diazepin-2-one
Pyrazolodiazepines
The pyrazolodiazepines were first outlined in 1969 in a patent and subsequent series of research papers published by a chemist at Parke-Davis by the name of Dr. Horace DeWald [94,95].
In total, only 5 of the compounds DeWald discovered showed psychoactivity in preliminary testing (razobazam, ripazepam, zomebazam, zolazepam, and zometapine).
Ultimately, only one of these medications, zolazepam, was developed for medical use.
Razobazam
Early research conducted on this atypical benzodiazepine drug in the 1980s reported nootropic-like effects in socially-deprived rats [93]. However, since this initial study, it appears there’s been no further development or formal interest in this compound.
This drug is rarely mentioned in the designer drug community as a possible mood-enhancer with distinct subjective differences from classical benzodiazepines. However, there’s no research whatsoever to back up this claim. At the moment, it’s unclear how this compound works.
Razobazam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 71228 |
| CAS#: | 78466-98-5 |
IUPAC Name: 3,8-dimethyl-4-phenyl-2H-pyrazolo[3,4-b][1,4]diazepine-5,7-dione
Ripazepam
Very little research was ever conducted on ripazepam, though initial studies suggest the drug had tranquilizing actions similar to other members of this class [96]. Safety studies were also conducted [97], but development was never completed.
The effects of this drug remain largely unknown, and ripazepam has not yet surfaced in the designer drug markets.
Ripazepam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 33474 |
| CAS#: | 26308-28-1 |
IUPAC Name: 1-ethyl-3-methyl-8-phenyl-4,6-dihydropyrazolo[4,3-e][1,4]diazepin-5-one
Zolazepam (Flupyrazapon)
Zolazepam (Flupyrazapon) is used as an anesthetic in veterinary medicine. It’s revered for its potency (roughly four times as potent as diazepam) and rapid onset of effects. It’s used primarily for tranquilizing larger animals like gorillas, bears, or elephants and is generally preferred to ketamine for having fewer side effects.
A combination of zolazepam and the dissociative anesthetic, tiletamine called Telazol is the most common form of this substance.
Zolazepam Specs:
| Status: | Approved (Veterinary Medicine) |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 35775 |
| CAS#: | 31352-82-6 |
IUPAC Name: 4-(2-fluorophenyl)-1,3,8-trimethyl-6H-pyrazolo[3,4-e][1,4]diazepin-7-one
Zomebazam
Zomebazam was patented in 1979 by the German pharmaceutical company Hoechst — but there are no indications this drug ever made it to market. It’s also unclear what the specific effects of this drug are, but due to structural similarities to razobazam and zometapine, it’s likely this drug maintains at least some of the conventional anxiolytic and sedative qualities inherent to this class.
Zomebazam has not yet appeared on the designer drug market in any significant quantities, and it’s unclear whether the subjective effects are desirable or not.
Zomebazam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 132677 |
| CAS#: | 78466-70-3 |
IUPAC Name: 1,3,8-trimethyl-4-phenylpyrazolo[3,4-b][1,4]diazepine-5,7-dione
Zometapine
Zometapine (CI-781) is an atypical benzodiazepine with characteristics of both the thienodiazepines and pyrazolodiazepines. This substance was studied as a potential antidepressant but was never developed into a final product. A study from 1984 suggested zometapine could be the first of a “new class of antidepressants” [98], but the project was killed shortly thereafter.
The psychoactivity of this drug is unknown, but early animal testing suggests the effects are not desirable as a recreational drug.
Zometapine Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 135410917 |
| CAS#: | 51022-73-2 |
IUPAC Name: 4-(3-chlorophenyl)-1,3-dimethyl-6,7-dihydro-2H-pyrazolo[3,4-e][1,4]diazepine
Pyrrolodiazepines
Pyrrolodiazepines are characterized by the fusion of a diazepine with a pyrrolidinedione. None of the drugs in this class ever made it into final development. They were too slow to kick in and aren’t as potent as other drugs in the benzodiazepine class.
The most significant of this group is a compound called premazepam, but even this compound is of little interest for both medical and recreational applications.
Premazepam
Premazepam is a slow-onset (about 2 hours) benzodiazepine research chemical. It has been shown in preliminary studies to possess benzodiazepine-like anxiolytic effects with comparable but weaker effects to diazepine. One study of interest found premazepam was distributed evenly throughout the brain, rather than concentrating in the white matter like other benzodiazepines [102].
Another unique trait of this drug is the lack of metabolism. 90% of the drug is eliminated as-is in the urine, and the remaining 10% of metabolites are completely inactive [99].
Like many benzodiazepines, this compound was discovered in the 1980s but never made it to market.
It’s rarely used as a recreational drug because of its slow onset time and low potency (roughly ⅓ the strength of diazepam).
Premazepam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Short-Acting |
| Common Dosage: | 25–50 mg |
| PubChem ID: | 72104 |
| CAS#: | 57435-86-6 |
IUPAC Name: 6,7-Dimethyl-5-phenyl-1,3-dihydropyrrolo[3,4-e][1,4]diazepin-2-one
Pyrrolobenzodiazepines
Most of the compounds in this class are atypical benzodiazepines that produce little to no activity on the GABA-A receptors. Most of these compounds are either antibiotics or anti-tumor in nature [100,101].
None of these compounds have psychoactive effects.
Examples of pyrrolobenzodiazepines include sibiromycin, tomaymycin, neothramycins, and DC-81.
Tetrahydroisoquinobenzodiazepines
The tetrahydroisoquinobenzodiazepine class combines benzodiazepine and tetrahydroisoquinoline skeletons [103]. This group has a lot of potential as new therapeutic agents due to the diverse binding affinities of both chemical groups.
Benzodiazepines are, of course, potent anxiolytics, hypnotics, muscle-relaxants, nootropics, and antibiotics.
The tetrahydroisoquinolines are a large and diverse group of pharmacologically-active alkaloids as well. Compounds such as papaverine (spasmolytic) and tubocurarine (myorelaxants) are well-known therapeutic agents in this class. Other prominent members include benzquinamide, tetrabenazine, and butaclamol.
There’s only one tetrahydroisoquinobenzodiazepine of interest currently — clazolam.
Clazolam
Clazolam (SAH-1123 or isoquinazepon) is a research chemical with anxiolytic and antidepressant qualities [103]. This drug was first discovered in the 1960s, but it’s unclear which company developed this drug as all the research has been removed from online databases and there are no patent entries for clazolam, SAH-1123, or isoquinazepon.
It’s likely that whoever designed this drug was interested in boosting the therapeutic value of benzodiazepines by fusing them with tetrahydroisoquinolines but failed to produce a drug that showed enough promise to invest the massive amounts of time and money necessary for bringing a drug to market.
Clazolam Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 24107 |
| CAS#: | 7492-29-7 |
IUPAC Name: 2-chloro-5-methyl-5,9,10,14b-tetrahydroisoquino[2,1-d][1,4]benzodiazepin-6(7H)-one
Benzodiazepine Prodrugs
A small selection of benzodiazepine prodrugs were developed in the 80s and 90s that aimed to produce an injectable (water-soluble) version of other benzodiazepine derivatives.
These prodrugs are inactive in their raw form but quickly metabolize into active benzodiazepines after coming into contact with enzymes in the blood or liver or the neutral pH of the blood.
Some of these prodrugs are sold as “legal” alternatives to benzodiazepines because their chemical structure is different enough to exclude them from the benzodiazepine class (and, therefore, the Schedule IV standing).
Alprazolam Triazolobenzophenone
Alprazolam triazolobenzophenone is a synthetic prod-drug for alprazolam (Xanax). A prodrug is a compound that is converted to the active material after it’s entered the body. In the case of alprazolam triazolobenzophenone, changes in pH cyclize the drug into alprazolam. When exposed to acidic conditions, the drug converts back to the prodrug form.
This drug was never developed or clinically tested, but it’s been on the designer drug market as an alternative to alprazolam since 2014 (Europe).
Alprazolam Triazolobenzophenone Specs:
| Status: | Research Chemical |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 15567865 |
| CAS#: | 69505-70-0 |
IUPAC Name: {2-[3-(Aminomethyl)-5-methyl-4H-1,2,4-triazol-4-yl]-5-chlorophenyl}(phenyl)methanone
Avizafone
Avizafone (Pro-Diazepam) is a water-soluble prodrug for diazepam (Valium) developed by Roche in the late 80s. It was designed as an injectable form of diazepine due to its water-soluble nature.
Most of the research on this drug explored its potential as an antidote for organophosphate poisoning [104]. It was believed to be a useful antidote for people exposed to nerve agents like sarin gas or atropine.
This drug is not used for its psychoactive or anxiolytic effects.
This drug rapidly converts to the active form (diazepam) as a result of enzymatic activation and deamination of metabolic byproducts in the neutral acidity of the blood (7.4) [105].
Avizafone Specs:
| Status: | Not Used |
| Duration of Effects: | Unknown |
| Common Dosage: | Unspecified |
| PubChem ID: | 71968 |
| CAS#: | 65617-86-9 |
IUPAC Name: (2S)-2,6-diamino-N-{[(2-benzoyl-4-chlorophenyl)methylcarbamoyl]methyl}hexanamide
Rilmazafone
Rilmazafone (Rhythmy) is a benzodiazepine prodrug developed in Japan. It’s water-soluble, allowing for greater bioavailability and enabling the drug to be injected. This molecule contains no diazepine ring and therefore isn’t technically classified as a benzodiazepine.
However, once ingested, the drug is metabolized by enzymes in the small intestines into active benzodiazepine metabolites, including 8-chloro-6-(2-chlorophenyl)-N,N-dimethyl-4H-1,2,4-triazolo [1,5-a][1,4]benzodiazepine-2-carboxamide [106]. The effects of this compound are reported to be highly similar to other benzodiazepine derivatives, including diazepam and alprazolam. The potency of this molecule is not well defined.
This drug is of interest to the designer drug market due to its ability to sidestep many of the regulations governing benzodiazepines around the world.
Rilmazafone Specs:
| Status: | Approved |
| Duration of Effects: | Short-Acting |
| Common Dosage: | Unspecified |
| PubChem ID: | 5069 |
| CAS#: | 99593-25-6 |
IUPAC Name: 1-[4-chloro-2-(2-chlorobenzoyl)phenyl]-5-[(glycylamino)methyl]-N,N-dimethyl-1H-1,2,4-triazole-3-carboxamide
Natural Benzodiazepines
The vast majority of benzodiazepines on the market today are synthetic molecules, but there are a few benzodiazepines that occur in nature. For example, some studies have found diazepam and N-desmethyldiazepam in trace amounts in wheat grains (0.2 ng/g) and the mammalian brain [108,109]. Delorazepam was found in Solanum tuberosum, Triticum aestivum, and Artemisia dracunculus, and nordazepam was discovered in Fagopyrum esculentum, and Glycine max.
There are also several plants that produce benzodiazepine-like effects but aren’t technically classified as benzos.
Let’s have a look.
Kava (Piper methysticum)
Kava (Piper methysticum) is a tropical plant species found on the scattered islands of the South Pacific. The kava plant doesn’t contain benzodiazepines, but the active ingredients, collectively known as the kavalactones, have benzodiazepine-like effects.
These compounds bind to the GABA-A receptors to induce a state of calmness and mild intoxication, just like benzodiazepines. Kava is traditionally used as a sleep aid, social lubrication, inebriant, and anxiolytic.
Unlike benzodiazepines, kava is unlikely to result in dependency. This is due to the complex nature of the plant and its active ingredients, which bind to GABA as well as a host of other receptors in the brain. In fact, the effects of repeated use are reversed compared to benzos. The more often you take kava, the stronger its effects. This is a concept called reverse tolerance — it’s something researchers are still seeking to understand.
Kava is not a direct alternative to benzos. It’s much milder. However, it’s a great option to consider instead of benzos, and some have used it to help alleviate similar issues, including anxiety, insomnia, and muscle tension.
Valerian (Valeriana officinalis)
Valerian root (Valeriana officinalis) contains a variety of compounds found to interact with the GABA receptors in a similar way to benzodiazepines [136].
The primary active ingredients in the plant are actinidine, chatinine, valerianine, valerine, alpha-methyl pyrryl ketone, and naphthyridin methyl ketone. Some of these compounds act as allosteric modulators to the GABA-A receptors; others interfere with the metabolism of GABA, leading to increased synaptic levels.
Much like other natural anxiolytics, valerian has a much lower chance of causing issues with dependency and addiction, even with long-term use.
In one animal study, a valerian extract showed a marked reduction in benzodiazepine withdrawal symptoms [137].
L-Theanine
L-Theanine is one of the active ingredients in green and black tea (Camellia sinensis). It’s classified as an amino acid with structural similarities to the neurotransmitter glutamate.
Glutamate is the most abundant excitatory neurotransmitter in the brain. When glutamate activity is high, we feel alert, anxious, and awake. GABA (the primary target for benzodiazepines) works in opposition to this neurotransmitter, causing us to feel relaxed, calm, and tired.
L-theanine works to reduce feelings of anxiety or stimulation by targeting both mechanisms — it increases GABA activity in a similar way as benzodiazepines, as well as decreasing the impact of glutamate by binding (but not activating) glutaminergic neurons in the brain.
Animal studies have found that L-theanine increases the concentrations of GABA, serotonin, and dopamine levels in the brain [138]. It’s commonly used as a natural anxiolytic, mild sedative, and nootropic.
It goes without saying, the effects of L-theanine are nowhere near as potent as benzodiazepines, but it’s a great alternative for people looking for gentle relief from anxiety or insomnia.
Why Do People Use Benzodiazepines?
Since the invention of the first benzodiazepine in the 1960s, over 2000 benzodiazepine derivatives have been developed. Only a small fraction of these drugs have ever been approved as medicine.
In the US, there are only 15 FDA-approved benzodiazepines on the market. Expanding to the global market, there are roughly 50 approved benzodiazepines.
Of the approved medications, the vast majority (90%) are prescribed for the treatment of anxiety or insomnia. Many of them are also used off-label as muscle relaxants.
In general, short-acting benzodiazepines are used for treating insomnia, and intermediate or long-acting benzodiazepines are used for managing anxiety. There are also stronger substances that are useful as an amnesic during surgery and a few long-acting benzos that are used to treat alcohol withdrawal.
NIDA published the results of a survey of over 5 million adults who misused benzodiazepines at least once over the past year. This study found the most common reason people take benzodiazepines was to help them relax or relieve tension (46.3%). Another 22% of people used benzos to help them sleep, and 11.8% took them to get high.
To Relieve Anxiety
Benzodiazepines have reigned as the king of the anxiety medications for decades. They replaced the more dangerous barbiturate class — offering stronger effects and less risk of overdose than their predecessor.
Benzos are seen as a chill pill — they chemically inhibit feelings of worry and anxiety. Most of the benzos used for treating anxiety kick in quickly, so people can take them at the first sign of a panic attack or episode.
Today, fewer doctors are prescribing benzodiazepines because of the high risk of abuse and addiction. They’re reserved for severe, short acute anxiety. They’re given to people who recently experienced a traumatic event or those on the verge of suicide.
These drugs certainly work to stop anxiety in its tracks, but they’ve proven to be a terrible long-term solution. In fact, the longer one takes benzos, the less effective they are. Abuse often leads to worse anxiety symptoms than users had before ever taking the drug in the first place.
The most common benzodiazepines prescribed for anxiety are Xanax (alprazolam), Ativan (lorazepam), Klonopin (clonazepam), Librium (chlordiazepoxide), Valium (diazepam), and Serax (oxazepam).
To Improve Sleep
The sedative qualities of benzodiazepines have made them a good candidate for inducing sleep in people suffering from insomnia. Unfortunately, most benzodiazepines reduce the amount of time spent in deep sleep, so users would often wake up feeling unrefreshed despite having been asleep all night.
Benzos like ProSom (estazolam), Halcion (triazolam), and Dormonoct (loprazolam) were commonly prescribed for insomnia from about the late 1960s to the mid-2000s, but they’ve largely been cast aside for a newer and safer class of drugs called the Z-drugs.
Z-drugs act like benzodiazepines but have less of an impact on deep sleep and are considered far less addictive overall.
To Get High
The third most common reason people take benzos is to get high. The effects of these drugs are very similar to other GABAergic substances, including GHB, alcohol, or phenibut.
In simple terms, benzos allow people to stop caring about anything and experience drunkenness comparable to alcohol until they eventually pass out.
These effects are desirable for many people.
People who take these drugs often claim it helps them feel more confident in social situations, experience less worry and inner turmoil, and allows them to release themselves from trying to meet the expectations of others.
Moderate doses of benzos can make speaking clearly or forming complex thoughts nearly impossible. High doses will often result in the user passing out and waking up with little to no memory of the previous night.
Unfortunately, these hypnotic qualities also make benzos a prime candidate as a date rape drug. Almost all benzos can have this effect, but the most commonly used are flunitrazepam and flunitrazolam.
Clinical Signs of Benzodiazepine Intoxication:
- Drowsiness/lethargy (65%)
- Slurred speech (17%)
- Confusion (14%)
- Agitation/irritability
- Ataxia (9%)
- Tachycardia (9%)
- Hypotension (8%)
- Comatose (4%)
- Bradycardia (4%)
(Source: Zawilska et al., 2019 [11])
In Combination With Other Substances
In the NIDA study, roughly 1.5% of people take benzos for the primary purpose of increasing or decreasing the effects of other drugs.
Benzodiazepines are often used alongside opiates to enhance the opiate high or by stimulant users to smooth the comedown. Others take benzos with alcohol to increase the intoxicating effects of the alcohol.
Mixing benzos with any of the substances mentioned above is extremely dangerous. Overdose is much, much more common when benzodiazepines are mixed with other drugs or alcohol. It’s this group of users that are leading the charge in terms of benzodiazepine overdose.
Adulteration
Many people use benzos mistakenly without them even knowing it too.
It’s common for someone to buy a pill they thought was another drug entirely that contained moderate to high levels of benzodiazepines instead.
A good example of this is the recent emergence of a drug combination referred to as “benzo dope.” Clandestine drug manufacturers and street-level drug dealers are mixing powerful benzodiazepines like alprazolam and bromazolam into painkillers or cocaine. They do this to increase the effects and improve their bottom line.
Benzo dope now accounts for half of all fentanyl samples collected in Toronto, Canada. Most people who take benzo dope have no idea the drugs they’re using even contain benzodiazepines.
How do Benzodiazepines Work?
The vast majority of benzodiazepines act as allosteric modulators of the GABA-A receptors. They don’t activate these receptors directly; rather, they alter their function to make the binding capacity of endogenous GABA stronger.
When GABA binds to the receptor, it causes a change in the polarity and action potential of the neuron. It makes it more difficult for electrical impulses to be transferred down the neuron to transmit messages around both the central and peripheral nervous systems.
The result of this action is an overall reduction in brain activity. This makes users feel less anxious and more sedated.
BZD Receptor Subtypes:
There are six receptor subtypes of the GABA-A receptors benzodiazepines can bind to. Each benzodiazepine has a different affinity for each of these receptors.
For example, drugs that bind to the α5 receptors produce stronger amnesic qualities, while drugs that bind to the α1 are generally going to perform better as anxiolytics.
Most benzodiazepines bind to all receptors (with the exception of α6) to some capacity but have a higher or lower affinity for specific subtypes.
α1 Receptors
The α1 receptor is the most predominant — accounting for roughly 60% of all GABA-A receptors [114]. These receptors are associated with the sedative, amnesic, and anticonvulsant action of benzodiazepine drugs [35].
These receptors are widespread throughout the central nervous system but are particularly concentrated in the cerebral cortex, hippocampus, amygdala, olfactory bulb, thalamus, basal forebrain, globus pallidus, substantia nigra pars reticulata, inferior colliculus, cerebellum, and brainstem.
α2 Receptors
The α2 receptors account for roughly 15–20% of the GABA-A receptors. They’re thought to play a major role in the use of benzodiazepines for treating psychiatric conditions like schizophrenia, depression, and pain disorders [134,135].
The α2 receptors are predominant in the cerebral cortex, hippocampal formation, amygdala, striatum, olfactory bulb, hypothalamus, superior colliculus, inferior olive, motor nuclei, and spinal cord dorsal horn [114].
α3 Receptors
Activation of the α3 receptors is believed to be specifically involved with anxiety [132].
These receptors are most highly concentrated in the cortex and reticular nucleus of the thalamus [133].
The thalamus is responsible for collecting all sensory information (with the exception of smell), organizing it, and transferring it to the cortex to be interpreted. It’s also involved with learning and memory, consciousness, wakefulness, and the regulation of sleep.
α4 Receptors
The α4 GABA-A receptor subtype accounts for less than 5% of all GABA-A receptors in the brain [114]. They’re most predominant in the dentate gyrus and thalamus regions of the brain.
Activation of the α4 receptors is associated with many of the intoxicating effects of alcohol and benzodiazepine drugs, such as muscle incoordination and ataxia [130]. It’s also believed to be largely responsible for the formation of tolerance and resistance to benzodiazepine drugs [131].
α5 Receptors
These receptors are most predominant in the hippocampus [129] but account for less than 5% of all GABA-A receptors.
The hippocampus plays a key role in learning and memory. It’s believed the activation of this receptor, in particular, is responsible for the amnesic effects of certain benzodiazepines.
Drugs like QH-II-66, flunitrazolam and flunitrazepam (as well as alcohol) have a high affinity for this receptor. Unsurprisingly, these compounds carry a particularly high risk of causing amnesia as a side effect (blackouts).
There are even some benzodiazepine derivatives that block this receptor, such as FG-8094, GL-II-73, PWZ-029, and RO4938581. These drugs are believed to act as nootropics because of this effect.
α6 Receptors
The α6 subtype accounts for less than 5% of all GABA-A receptors in the human brain [114]. They’re most concentrated in the cerebellum and dorsal cochlear nucleus.
Classical benzodiazepines do not interact with these receptors, and they are not believed to be associated with any of the pharmacological profiles of benzodiazepine drugs.
Benzodiazepine Addiction: A Legitimate Concern
Addiction to benzos doesn’t happen overnight — it tends to sneak up on people if they aren’t careful.
At first, the effects of the drug are positive — they help people feel calmer, more relaxed, and less focused on the most salient issues in one’s life.
Addiction only appears once people have been using the drug for a while. The more frequently the drug is used, the faster the body starts to become tolerant and eventually dependent on the effects of the drug.
Tolerance forms when the body anticipates the effects of the drug. In order to maintain balance, it will resist the effects of the drug, so the user needs to increase the dose to get the same level of effects.
With repeated use, this tolerance starts to overcompensate, making it difficult to maintain balance without taking the drug. This is called dependence because the user is dependent on the drug in order to feel “normal.”
People dependent on benzodiazepines feel withdrawal symptoms whenever the drug wears off. Withdrawal side effects are essentially the exact opposite of what the drug is supposed to produce (severe anxiety, insomnia, and muscle tension).
This is the trap: once someone is dependent on benzodiazepines, it becomes increasingly difficult to quit. Stopping the drug can bring debilitating anxiety and panic attacks, it makes sleeping almost impossible, and users feel extensive muscle pain and tension.
Benzodiazepines are one of the most challenging drugs to quit because of how severe the withdrawal symptoms are. It’s also why there are so many warnings against using any of these drugs habitually for any reason.
Benzodiazepine Withdrawals
There are only two forms of withdrawal that can be fatal if medical care isn’t given — this is alcohol and benzodiazepines — both for the same reason.
Tolerance to benzos involves a combination of reduced GABA receptor activity and increased glutamate activity. Sudden withdrawal from these compounds can result in an inability of the neurological system to regulate hyperactive states.
Excessive glutamate levels left unchecked by GABA can lead to a condition called excitotoxicity. This can lead to widespread neuronal damage, leading to seizures, coma, and death.
The majority of withdrawal symptoms last 2–6 weeks, depending on how slowly the drugs are weaned off. However, lingering effects of benzo withdrawal can remain in place for months or even years after quitting. These include symptoms such as anxiety, insomnia, and muscle spasms. Long-term withdrawal symptoms are reported in roughly 1 in 10 people formerly addicted to benzodiazepines.
Benzodiazepine Withdrawal Symptoms Include:
- Agitation
- Delirium tremens
- Depersonalization
- Depression
- Derealization
- Fearfulness
- Gastric problems
- Hypersensitivity to stimuli
- Insomnia
- Irritability
- Muscle spasms
- Psychosis
- Seizures
- Suicidal behavior
- Sweating
- Tremors
A Brief History of Benzodiazepines
The first benzodiazepine was discovered nearly 70 years ago in 1955 by an American chemist named Leo Sternbach [50]. The drug, chlordiazepoxide, was later sold under the brand name Librium in 1960 by Hoffmann-La Roche.
Just three years later, the next benzodiazepine was released by the same company. This was diazepam (Valium), which quickly went on to become one of the most widely sold medications on Earth. By 1977 benzodiazepines as a whole earned the title of the most commonly-prescribed medication class on Earth.
This class of drug became popular for three reasons:
- Benzos were far less dangerous than the previous generation of drugs, called barbiturates.
- Benzos offered treatment for some of the most common health conditions in the world — anxiety, depression, muscle pain, and insomnia.
- Benzos were believed to be less addictive than barbiturates [123].
Benzodiazepines remained highly popular for over a decade before the mechanism of action was fully understood. By then, it was clear this class of drugs was more dangerous than previously thought. In the late 1980s, many doctors were campaigning for stronger restrictions on this class, and new warning labels were implemented on the packaging.
The first designer benzodiazepine drugs began showing up in the 2000s — starting with phenazepam in 2007 and etizolam several years later in 2011. Since then, hundreds of designer benzodiazepines have entered the market. When regulators cracked down on one compound and took measures to ban it, three more would step into its place. This game of cat and mouse continues to this day.
Today, benzodiazepines are still widely used, but doctors are much more conservative in prescribing them. There are also more protocols in place to prevent people from using these drugs long-term, such as monitoring pickups at pharmacies and inter-state prescriptions. It’s the long-term use of these drugs where the vast majority of problems arise.
The designer drug market for benzodiazepines is thriving as a result of these crackdowns. People who are addicted to benzodiazepines from a past prescription now buy them online or on the street to prevent withdrawal and keep anxiety or insomnia symptoms at bay.
Others use benzos for their inebriating effects, which is comparable to the disinhibition induced by alcohol, with additional feelings of euphoria and relaxation. People often take benzos to cut the side effects of stimulants like cocaine or methamphetamine or with depressants like opiates or alcohol to numb themselves and block out psychological pain or torment.
Benzodiazepine FAQs
1. What Are the Side Effects of Benzodiazepines?
The most common side effect of benzodiazepines is sedation and poor concentration. Long-term use carries a far greater risk of more serious side effects like lapses in memory, problems with libido, and reduced cognitive capacity.
Side effects of benzodiazepines include:
- Drowsiness
- Dizziness
- Decreased alertness
- Poor concentration
- Lack of coordination
- Decreased libido
2. What Is The Strongest Benzodiazepine?
The triazolobenzodiazapine class contains several highly potent benzodiazepine derivatives. Most of the strongest benzos on the market today are in this subgroup.
The strongest benzodiazepines known to date:
- Flunitrazolam (Designer Drug)
- Brotizolam (Lendormin)
- Flubromazolam (JYI-73)
- Triazolam (Halcion)
- Lormetazepam (Noctamid)
- Alprazolam (Xanax)
- Clonazolam (Designer Drug)
- Lorazepam (Ativan)
- Estazolam (Etilaam)
- Flunitrazepam (Rohypnol)
3. What is a Half-Life?
Half-life (t1⁄2) refers to the amount of time for a substance to be reduced to exactly half its original value. This measurement is used to determine how long a substance will remain active in the body.
When talking about drugs, biological half-life (AKA elimination half-life) refers specifically to the amount of time it takes for the drug to go from its maximum plasma (blood) concentrations to exactly half the maximum value.
Factors like metabolic rate, protein binding, and receptor interactions can all affect the biological half-life of a drug.
A half-life is a form of exponential decay, which means the rate of elimination is proportional to the current value. So if a substance has a half-life of 1 hour, it means that exactly 50% of the drug is removed; 1 hour later, half of the remaining amount is removed (25%), then half of that (12.5%), then half of that (6.12%), and so-on.
4. How Long Are Benzodiazepines Detectable in Urine?
The amount of time benzodiazepines remain in the urine depends on the half-life of the drug. Short-acting benzodiazepines like triazolam (Halcion), estazolam (ProSom), and zolpidem (Ambien) are removed from the body quickly and are usually undetectable in the urine within 48 hours.
Intermediate-acting benzodiazepines such as alprazolam (Xanax), clobazam (Onfi), or lorazepam (Ativan) may remain detectable for 2–5 days.
Long-acting benzodiazepines such as chlordiazepoxide (Librium), diazepam (Valium), and flutoprazepam (Restas) may remain detectable for as long as seven days.
5. What Are Some Alternatives to Benzodiazepines?
Popular benzodiazepine alternatives for managing anxiety include herbs like kava or valerian, GABA supplements, L-theanine, or phenibut. Barbiturates offer similar effects but are no longer used due to the high risk of overdose.
Popular alternatives to benzodiazepines for improving sleep include the newer class of Z-drugs like eszopiclone (Lunesta), zaleplon (Sonata), and zolpidem (Ambien).
6. Is There An Antidote For Benzodiazepines?
Yes. The primary antidote used for managing benzodiazepine toxicity is flumazenil (Anexate).
This drug is itself a benzodiazepine derivative, but it acts as a nonspecific competitive antagonist and the BZD receptors. It works by binding to the receptors to block active benzodiazepines from binding instead.
7. Which Demographics Use Benzos The Most?
According to a Swedish study published in 2018, 81% of patients hospitalized for benzodiazepine intoxication were male [12].
These findings are supported by the National Poison Data System, which reported that 86% of patients hospitalized for DBZD (designer benzodiazepine drug) use from 2014–2017 were male.
8. What Should I Do If I’m Addicted To Benzodiazepines?
Addiction to benzodiazepines is common. If you or someone you know is addicted to their anxiety or sleep medications, help is available.
The first step is to reach out to an addiction hotline or speak to your doctor directly. There is a lot of help available; you just have to ask for it.
The withdrawals from benzodiazepine addiction can be dangerous, so it’s important to consult with a medical professional before attempting it on your own.
Addiction hotlines:
- USA — 1 800 662 4357
- Canada — 1 866 585 0445
- United Kingdom — 0300 999 1212
If you are in an emergency situation, call 911 or go to your local emergency department immediately.
References
- Ashton, H. (2005). The diagnosis and management of benzodiazepine dependence. Current opinion in Psychiatry, 18(3), 249-255.
- Dodds, T. J. (2017). Prescribed benzodiazepines and suicide risk: a review of the literature. The primary care companion for CNS disorders, 19(2), 22746.
- Zhong, G., Wang, Y., Zhang, Y., & Zhao, Y. (2015). Association between benzodiazepine use and dementia: a meta-analysis. PloS one, 10(5), e0127836.
- Nakafero, G., Sanders, R. D., Nguyen-Van-Tam, J. S., & Myles, P. R. (2016). The association between benzodiazepines and influenza-like illness-related pneumonia and mortality: a survival analysis using UK Primary Care data. Pharmacoepidemiology and Drug Safety, 25(11), 1263-1273.
- Kim, H. B., Myung, S. K., Park, Y. C., & Park, B. (2017). Use of benzodiazepine and risk of cancer: A meta-analysis of observational studies. International Journal of Cancer, 140(3), 513-525.
- Fraser, A. D. (1998). Use and abuse of the benzodiazepines. Therapeutic drug monitoring, 20(5), 481-489.
- Bachhuber, M. A., Hennessy, S., Cunningham, C. O., & Starrels, J. L. (2016). Increasing benzodiazepine prescriptions and overdose mortality in the United States, 1996–2013. American journal of public health, 106(4), 686-688.
- Marin, M. J., & van Wijk, X. M. R. (2019). The evolution of designer benzodiazepines: challenges for detection and monitoring. Clinical Laboratory News: Washington, DC.
- Perez Orts, M., van Asten, A., & Kohler, I. (2022). The Evolution Toward Designer Benzodiazepines in Drug-Facilitated Sexual Assault Cases. Journal of Analytical Toxicology.
- EMCDDA. (2018). The misuse of benzodiazepines among high-risk opioid users in Europe. Perspectives on Drugs, 2018.
- Zawilska, J. B., & Wojcieszak, J. (2019). An expanding world of new psychoactive substances—designer benzodiazepines. Neurotoxicology, 73, 8-16.
- Bäckberg, M., Pettersson Bergstrand, M., Beck, O., & Helander, A. (2019). Occurrence and time course of NPS benzodiazepines in Sweden–results from intoxication cases in the STRIDA project. Clinical Toxicology, 57(3), 203-212.
- Carpenter, J. E., Murray, B. P., Dunkley, C., Kazzi, Z. N., & Gittinger, M. H. (2019). Designer benzodiazepines: a report of exposures recorded in the National Poison Data System, 2014–2017. Clinical toxicology, 57(4), 282-286.
- Calcaterra, N. E., & Barrow, J. C. (2014). Classics in chemical neuroscience: diazepam (valium). ACS chemical neuroscience, 5(4), 253-260.
- A. Winkler, D., R. Burden, F., & Watkins, A. J. (1998). Atomistic topological indices applied to benzodiazepines using various regression methods. Quantitative Structure‐Activity Relationships, 17(01), 14-19.
- Babbini, M., Torrielli, M. V., Strumia, E., Gaiardi, M., Bartoletti, M., & De Marchi, F. (1975). Sedative-hypnotic properties of a new benzodiazepine in comparison with flurazepam. Pharmacological and clinical findings. Arzneimittel-forschung, 25(8), 1294-1300.
- Baile, C. A., & McLaughlin, C. L. (1979). A review of the behavioral and physiological responses to elfazepam, a chemical feed intake stimulant. Journal of Animal Science, 49(5), 1371-1395.
- Van Den Broek, G. W., Robertson, J., Keim, D. A., & Baile, C. A. (1979). Feeding and depression of abomasal secretion in sheep elicited by elfazepam and 9-aza-cannabinol. Pharmacology Biochemistry and Behavior, 11(1), 51-56.
- Sakai, Y., & Namima, M. (1985). Inhibitory effect on 3H-diazepam binding and potentiating action on GABA of ethyl loflazepate, a new minor tranquilizer. The Japanese Journal of Pharmacology, 37(4), 373-379.
- Davi, H., Guyonnet, J., Sales, Y., & Cautreels, W. (1985). Metabolism of ethyl loflazepate in the rat, the dog, the baboon and in man. Arzneimittel-forschung, 35(7), 1061-1065.
- Baumeister, D., Tojo, L. M., & Tracy, D. K. (2015). Legal highs: staying on top of the flood of novel psychoactive substances. Therapeutic advances in psychopharmacology, 5(2), 97-132.
- Nakatsuka, I., Shimizu, H., Asami, Y., Katoh, T., Hirose, A., & Yoshitake, A. (1985). Benzodiazepines and their metabolites: relationship between binding affinity to the benzodiazepine receptor and pharmacological activity. Life sciences, 36(2), 113-119.
- Shimamine, M., Masunari, T., & Nakahara, Y. (1993). Studies on identification of drugs of abuse by diode array detection. I. Screening-test and identification of benzodiazepines by HPLC-DAD with ICOS software system. Eisei Shikenjo hokoku. Bulletin of National Institute of Hygienic Sciences, (111), 47-56.
- Sukamoto, T., Aikawa, K., Itoh, K., & Nose, T. (1980). Psycopharmacological and general pharmacological studies of 7-chloro-1-cyclopropylmethyl-1, 3-dihydro-5-(2-fluorophenyl)-2H-1, 4-benzodiazepin-2-one (KB-509)(author’s transl). Nihon Yakurigaku zasshi. Folia Pharmacologica Japonica, 76(6), 447-468.
- Miyaguchi, H., Kuwayama, K., Tsujikawa, K., Kanamori, T., Iwata, Y. T., Inoue, H., & Kishi, T. (2006). A method for screening for various sedative-hypnotics in serum by liquid chromatography/single quadrupole mass spectrometry. Forensic Science International, 157(1), 57-70.
- Clarke, C. H., Ferres, H. M., Nicholson, A. N., & Stone, B. M. (1975). Proceedings: Effect of diazepam and a soluble salt of diazepam (fosazepam) on sleep in man. British Journal of Pharmacology, 55(2), 262P.
- Viukari, M., Linnoila, M., & Aalto, U. (1978). Efficacy and side effects of flurazepam, fosazepam, and nitrazepam as sleeping aids in psychogeriatric patients. Acta Psychiatrica Scandinavica, 57(1), 27-35.
- Andronati, S. A., Zin’kovskiĭ, V. G., MIu, T., Stankevich, E. A., & Zhuk, O. V. (1992). Biokinetics of a new prodrug gidazepam and its metabolite. Biulleten’Eksperimental’noi Biologii i Meditsiny, 113(1), 45-47.
- Zherdev, V. P., Neznamov, G. G., Kolyvanov, G. B., Litvin, A. A., & Otabekova, S. G. (1993). The pharmacokinetic aspects of the clinical action of gidazepam. Eksperimental’naia i Klinicheskaia Farmakologiia, 56(3), 50-52.
- Anhalt, H. S., Young, R., & Roginsky, M. (1980). Double-blind comparison of ketazolam, diazepam and placebo in once-a-day vs tid dosing. The Journal of Clinical Psychiatry.
- O’Boyle, C., Lambe, R., & Darragh, A. (1985). Central effects in man of the novel schistosomicidal benzodiazepine meclonazepam. European journal of clinical pharmacology, 29(1), 105-108.
- Gielsdorf, W., Molz, K. H., Hausleiter, H. J., Achtert, G., & Philipp, P. (1986). Pharmacokinetic profile of metaclazepam (Talis®), a new 1.4-benzodiazepine. European journal of drug metabolism and pharmacokinetics, 11(3), 205-210.
- Nikaido, A. M., & Ellinwood, E. H. (1987). Comparison of the effects of quazepam and triazolam on cognitive-neuromotor performance. Psychopharmacology, 92(4), 459-464.
- Manchester, K. R., Maskell, P. D., & Waters, L. (2018). Experimental versus theoretical log D7. 4, pKa and plasma protein binding values for benzodiazepines appearing as new psychoactive substances. Drug Testing and Analysis, 10(8), 1258-1269.
- Möhler, H., Fritschy, J. M., & Rudolph, U. (2002). A new benzodiazepine pharmacology. Journal of Pharmacology and Experimental Therapeutics, 300(1), 2-8.
- Janbroers, J. M. (1984). Pinazepam: review of pharmacological properties and therapeutic efficacy. Clinical Therapeutics, 6(4), 434-450.
- Roth, T., Tietz, E. I., Kramer, M., & Kaffeman, M. (1979). The effect of a single dose of quazepam (Sch-16134) on the sleep of chronic insomniacs. Journal of International Medical Research, 7(6), 583-587.
- Ankier, S. I., & Goa, K. L. (1988). Quazepam. Drugs, 35(1), 42-62.
- Obradović, A. L., Joksimović, S., Poe, M. M., Ramerstorfer, J., Varagic, Z., Namjoshi, O., … & Savić, M. M. (2014). Sh-I-048A, an in vitro non-selective super-agonist at the benzodiazepine site of GABAA receptors: the approximated activation of receptor subtypes may explain behavioral effects. Brain research, 1554, 36-48.
- Clayton, T., Poe, M. M., Rallapalli, S., Biawat, P., Savić, M. M., Rowlett, J. K., … & Cook, J. M. (2015). A review of the updated pharmacophore for the alpha 5 GABA (A) benzodiazepine receptor model. International journal of medicinal chemistry, 2015.
- Römer, D., Büscher, H. H., Hill, R. C., Maurer, R., Petcher, T. J., Zeugner, H., … & Thies, P. W. (1982). Unexpected opioid activity in a known class of drug. Life Sciences, 31(12-13), 1217-1220.
- Peng, G. W. (1984). Assay of adinazolam in plasma by liquid chromatography. Journal of pharmaceutical sciences, 73(8), 1173-1175.
- Jacob, S., Veenstra-VanderWeele, J., Murphy, D., McCracken, J., Smith, J., Sanders, K., … & Anagnostou, E. (2022). Efficacy and safety of balovaptan for socialisation and communication difficulties in autistic adults in North America and Europe: a phase 3, randomised, placebo-controlled trial. The Lancet Psychiatry, 9(3), 199-210.
- Sys, J., Van Cleynenbreugel, S., Deschodt, M., Van der Linden, L., & Tournoy, J. (2020). Efficacy and safety of non-benzodiazepine and non-Z-drug hypnotic medication for insomnia in older people: a systematic literature review. European Journal of Clinical Pharmacology, 76(3), 363-381.
- Hester Jr, J. B., Rudzik, A. D., & Kamdar, B. V. (1971). 6-Phenyl-4H-s-triazolo [4, 3-a][1, 4] benzodiazepines which have central nervous system depressant activity. Journal of medicinal chemistry, 14(11), 1078-1081.
- Miura, M., Otani, K., & Ohkubo, T. (2005). Identification of human cytochrome P450 enzymes involved in the formation of 4-hydroxyestazolam from estazolam. Xenobiotica, 35(5), 455-465.
- Moosmann, B., King, L. A., & Auwärter, V. (2015). Designer benzodiazepines: a new challenge. World Psychiatry, 14(2), 248.
- Łukasik-Głębocka, M., Sommerfeld, K., Teżyk, A., Zielińska-Psuja, B., Panieński, P., & Żaba, C. (2016). Flubromazolam–A new life-threatening designer benzodiazepine. Clinical Toxicology, 54(1), 66-68.
- Ameline, A., Richeval, C., Gaulier, J. M., Raul, J. S., & Kintz, P. (2018). Characterization of flunitrazolam, a new designer benzodiazepine, in oral fluid after a controlled single administration. Journal of Analytical Toxicology, 42(6), e58-e60.
- Sternbach, L. H. (1979). The benzodiazepine story. Journal of medicinal chemistry, 22(1), 1-7.
- Moosmann, B., Hutter, M., Huppertz, L. M., Ferlaino, S., Redlingshöfer, L., & Auwärter, V. (2013). Characterization of the designer benzodiazepine pyrazolam and its detectability in human serum and urine. Forensic toxicology, 31(2), 263-271.
- Hrib, N. J., & Martin, L. L. (1989). Antianxiety Agents and Anticonvulsants. In Annual Reports in Medicinal Chemistry (Vol. 24, pp. 11-20). Academic Press.
- Krátký, M., Svrčková, K., Vu, Q. A., Štěpánková, Š., & Vinšová, J. (2021). Hydrazones of 4-(Trifluoromethyl) benzohydrazide as New Inhibitors of Acetyl-and Butyrylcholinesterase. Molecules, 26(4), 989.
- Müller, W. E., Groh, B., Bub, O., Hofmann, H. P., & Kreiskott, H. (1986). In vitro and in vivo studies of the mechanism of action of arfendazam, a novel 1, 5-benzodiazepine. Pharmacopsychiatry, 19(04), 314-315.
- Donevan, S. D., & Rogawski, M. A. (1998). Allosteric regulation of α-amino-3-hydroxy-5-methyl-4-isoxazole-propionate receptors by thiocyanate and cyclothiazide at a common modulatory site distinct from that of 2, 3-benzodiazepines. Neuroscience, 87(3), 615-629.
- Donevan, S. D., & Rogawski, M. A. (1993). GYKI 52466, a 2, 3-benzodiazepine, is a highly selective, noncompetitive antagonist of AMPA/kainate receptor responses. Neuron, 10(1), 51-59.
- Horváth, K., Andrási, F., Berzsenyi, P., Pátfalusi, M., Patthy, M., Szabó, G., … & Botka, P. (1989). A new psychoactive 5H-2, 3-benzodiazepine with a unique spectrum of activity. Arzneimittel-forschung, 39(8), 894-899.
- Bond, A., & Lader, M. (1982). A comparison of the psychotropic profiles of tofisopam and diazepam. European Journal of Clinical Pharmacology, 22(2), 137-142.
- Qneibi, M., Jaradat, N., Hawash, M., Olgac, A., & Emwas, N. (2020). Ortho versus meta chlorophenyl-2, 3-benzodiazepine analogues: synthesis, molecular modeling, and biological activity as AMPAR antagonists. ACS omega, 5(7), 3588-3595.
- Ates-Alagoz, Z., & Adejare, A. (2017). Physicochemical Properties for Potential Alzheimer’s Disease Drugs. Drug Discovery Approaches for the Treatment of Neurodegenerative Disorders, 59-82.
- Zhang, P., Zhang, W., Liu, R., Harris, B., Skolnick, P., & Cook, J. M. (1995). Synthesis of novel imidazobenzodiazepines as probes of the pharmacophore for” diazepam-insensitive” GABAA receptors. Journal of medicinal chemistry, 38(10), 1679-1688.
- Giusti, P., Guidetti, G., Costa, E., & Guidotti, A. (1991). The preferential antagonism of pentylenetetrazole proconflict responses differentiates a class of anxiolytic benzodiazepines with potential antipanic action. Journal of Pharmacology and Experimental Therapeutics, 257(3), 1062-1068.
- Nicholson, A. N. (1979). Differential effects of the 1, 4 and 1, 5 benzodiazepines on performance in healthy man. British journal of clinical pharmacology, 7(S1), 83S-84S.
- Schukin, S. I., Zinkovsky, V. G., & Zhuk, O. V. (2011). Elimination kinetics of the novel prodrug cinazepam possessing psychotropic activity in mice. Pharmacological Reports, 63(5), 1093-1100.
- Zakusov, V. V. (1983). Pharmacology of uxepam. Farmakologiya i Toksikologiya.
- Ebenezer, I. S., De La Riva, C., & Baldwin, B. A. (1990). Effects of the CCK receptor antagonist MK-329 on food intake in pigs. Physiology & Behavior, 47(1), 145-148.
- Barnett, A., & RI, T. (1974). The pharmacology of fletazepam a centrally-acting muscle relaxant.
- Rice, K. C., Brossi, A., TALLMAN, J., Paul, S. M., & Skolnick, P. (1979). Irazepine, a noncompetitive, irreversible inhibitor of 3H-diazepam binding to benzodiazepine receptors. Nature, 278(5707), 854-855.
- Auta, J., Costa, E., Davis, J. M., & Guidotti, A. (2005). Imidazenil: An antagonist of the sedative but not the anticonvulsant action of diazepam. Neuropharmacology, 49(3), 425-429.
- Quirk, K., Blurton, P., Fletcher, S., Leeson, P., Tang, F., Mellilo, D., … & McKernan, R. M. (1996). [3H] L-655,708, a novel ligand selective for the benzodiazepine site of GABAA receptors which contain the α5 subunit. Neuropharmacology, 35(9-10), 1331-1335.
- Chambers, M. S., Atack, J. R., Broughton, H. B., Collinson, N., Cook, S., Dawson, G. R., … & MacLeod, A. M. (2003). Identification of a novel, selective GABAA α5 receptor inverse agonist which enhances cognition. Journal of medicinal chemistry, 46(11), 2227-2240.
- Fischell, J., Van Dyke, A. M., Kvarta, M. D., LeGates, T. A., & Thompson, S. M. (2015). Rapid antidepressant action and restoration of excitatory synaptic strength after chronic stress by negative modulators of alpha5-containing GABAA receptors. Neuropsychopharmacology, 40(11), 2499-2509.
- Savić, M. M., Clayton, T., Furtmüller, R., Gavrilović, I., Samardžić, J., Savić, S., … & Cook, J. M. (2008). PWZ-029, a compound with moderate inverse agonist functional selectivity at GABAA receptors containing α5 subunits, improves passive, but not active, avoidance learning in rats. Brain research, 1208, 150-159.
- Antonik, L. J., Goldwater, D. R., Kilpatrick, G. J., Tilbrook, G. S., & Borkett, K. M. (2012). A placebo-and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): Part I. Safety, efficacy, and basic pharmacokinetics. Anesthesia & Analgesia, 115(2), 274-283.
- Dingemanse, J., Van Gerven, J. M. A., Schoemaker, R. C., Roncari, G., Oberyé, J. J. L., Van Oostenbruggen, M. F., … & Cohen, A. F. (1997). Integrated pharmacokinetics and pharmacodynamics of Ro 48–6791, a new benzodiazepine, in comparison with midazolam during first administration to healthy male subjects. British journal of clinical pharmacology, 44(5), 477-486.
- Ballard, T. M., Knoflach, F., Prinssen, E., Borroni, E., Vivian, J. A., Basile, J., … & Hernandez, M. C. (2009). RO4938581, a novel cognitive enhancer acting at GABAA α5 subunit-containing receptors. Psychopharmacology, 202(1), 207-223.
- Martínez-Cué, C., Martínez, P., Rueda, N., Vidal, R., García, S., Vidal, V., … & Hernández, M. C. (2013). Reducing GABAA α5 receptor-mediated inhibition rescues functional and neuromorphological deficits in a mouse model of down syndrome. Journal of Neuroscience, 33(9), 3953-3966.
- López-Romero, B., Evrard, G., Durant, F., Sevrin, M., & George, P. (1998). Molecular structure and stereoelectronic properties of sarmazenil—A weak inverse agonist at the omega modulatory sites (benzodiazepine receptors): Comparison with bretazenil and flumazenil. Bioorganic & medicinal chemistry, 6(10), 1745-1757.
- Ito, M., Miyajima, T., Fujii, T., & Okuno, T. (2004). Cloxazolam treatment for patients with intractable epilepsy. Pediatric neurology, 30(2), 111-114.
- Sakai, Y. (1983). Comparative study on the effects of haloxazolam and estazolam, new sleep inducing drugs, on the α-and γ-motor systems. Japanese journal of pharmacology, 33(5), 1017-1025.
- Hultborn, H., & Fedirchuk, B. (2009). Spinal motor neurons: properties. Encyclopedia of Neuroscience, 9, 309-319.
- OCHS, H. R., Greenblatt, D. J. , Verburg-Ochs, B. T., and Locniskar, A. (1984). Comparative single-dose kinetics of oxazolam, prazepam, and clorazepate: three precursors of desmethyldiazepam. The Journal of Clinical Pharmacology, 24(10), 446-451.
- Mariño, E. L., & Dominguez-Gil, A. (1986). Pharmacokinetics of tiadipone: a new anxiolytic. International Journal of Clinical Pharmacology, Therapy, and Toxicology, 24(9), 482-484.
- Langley, M. S., & Clissold, S. P. (1988). Brotizolam. Drugs, 35(2), 104-122.
- Nakazawa, Y., Kotorii, M., Ohshima, M., Horikawa, S., & Tachibana, H. (1975). Effects of thienodiazepine derivatives on human sleep as compared to those of benzodiazepine derivatives. Psychopharmacologia, 44(2), 165-171.
- Catalani, V., Floresta, G., Botha, M., Corkery, J. M., Guirguis, A., Vento, A., … & Schifano, F. (2022). In silico studies on recreational drugs: 3D‐QSAR prediction of classified and de novo designer benzodiazepines. Chemical Biology & Drug Design.
- Takehara, S., Mikashima, H., Muramoto, Y., Terasawa, M., Setoguchi, M., & Tahara, T. (1990). Pharmacological actions of Y-24180, a new specific antagonist of platelet activating factor (PAF): II. Interactions with PAF and benzodiazepine receptors. Prostaglandins, 40(6), 571-583.
- Komatsu, H., Amano, M., Yamaguchi, S., & Sugahara, K. (1999). Inhibition of activation of human peripheral blood eosinophils by Y-24180, an antagonist to platelet-activating factor receptor. Life sciences, 65(13), PL171-PL176.
- Zhang, W., & Bymaster, F. P. (1999). The in vivo effects of olanzapine and other antipsychotic agents on receptor occupancy and antagonism of dopamine D1, D2, D3, 5HT2A and muscarinic receptors. Psychopharmacology, 141(3), 267-278.
- Wang, J. S., Zhu, H. J., Markowitz, J. S., Donovan, J. L., & DeVane, C. L. (2006). Evaluation of antipsychotic drugs as inhibitors of multidrug resistance transporter P-glycoprotein. Psychopharmacology, 187(4), 415-423.
- Johnson, D. E., Yamazaki, H., Ward, K. M., Schmidt, A. W., Lebel, W. S., Treadway, J. L., … & Rollema, H. (2005). Inhibitory effects of antipsychotics on carbachol-enhanced insulin secretion from perifused rat islets: role of muscarinic antagonism in antipsychotic-induced diabetes and hyperglycemia. Diabetes, 54(5), 1552-1558.
- Fabian, A., Röhmel, R., & Kubicki, S. (1984). Changes in the length of sleep cycles during administration of flurazepam and lopirazepam. EEG-EMG Zeitschrift fur Elektroenzephalographie, Elektromyographie und Verwandte Gebiete, 15(3), 151-158.
- Hock, F. J., & Scheich, H. (1986). Functional activity in the brain of socially deprivated rats produced by an active avoidance test after razobazam (Hoe 175) treatment: a 2-deoxyglucose study. Behavioral and neural biology, 46(3), 398-409.
- DeWald, H. A., Nordin, I. C., L’Italien, Y. J., & Parcell, R. F. (1973). Pyrazolodiazepines. 1, 3-(and 2, 3-) Dialkyl-4, 6-dihydro-8-arylpyrazolo [4, 3-e][1, 4] diazepin-5-ones as antianxiety agents. Journal of Medicinal Chemistry, 16(12), 1346-1354.
- DeWald, H. A., Lobbestael, S., & Butler, D. E. (1977). Pyrazolodiazepines. 2. 4-Aryl-1, 3-dialkyl-6, 8-dihydropyrazolo [3, 4-e][1, 4] diazepin-7 (1H)-ones as antianxiety and anticonvulsant agents. Journal of Medicinal Chemistry, 20(12), 1562-1569.
- Baraldi, P. G., Manfredini, S., Periotto, V., Simoni, D., Guarneri, M., & Borea, P. A. (1985). Synthesis and Interaction of 5-(Substituted-phenyl)-3-methy1-6, 7-dihydropyrazolo [4, 3-e][1, 4] diazepin-8 (7H)-ones with Benzodiazepine Receptors in Rat Cerebral Cortex. Journal of medicinal chemistry, 28(5), 683-685.
- Fitzgerald, J. E., de la Iglesia, F. A., & McGuire, E. J. (1984). Carcinogenicity studies in rodents with ripazepam, a minor tranquilizing agent. Toxicological Sciences, 4(2part1), 178-190.
- Katz, R. J. (1984). Effects of zometapine, a structurally novel antidepressant, in an animal model of depression. Pharmacology Biochemistry and Behavior, 21(4), 487-490.
- Vitiello, B. (1984). Pharmacokinetics and metabolism of premazepam, a new potential anxiolytic, in humans. International Journal of Clinical Pharmacology, Therapy & Toxicology.
- Gerratana, B. (2012). Biosynthesis, synthesis, and biological activities of pyrrolobenzodiazepines. Medicinal research reviews, 32(2), 254-293.
- Hartley, J. A. (2011). The development of pyrrolobenzodiazepines as antitumour agents. Expert opinion on investigational drugs, 20(6), 733-744.
- Zanolo, G., Giachetti, M. C., Canali, S., Bernareggi, A., Tarzia, G., & Assandri, A. (1986). Disposition of premazepam, an anti-anxiety pyrrolodiazepine, in the cynomolgus monkey. European journal of drug metabolism and pharmacokinetics, 11(2), 151-157.
- Potikha, L. M., & Kovtunenko, V. A. (2009). Synthesis and properties of 3-aminodihydroisoquinolines. Russian Chemical Reviews, 78(6), 513.
- Karlsson, B., Lindgren, B., Millquist, E., Sandberg, M., & Sellström, Å. (1990). On the use of diazepam and pro-diazepam (2-benzoyl-4-chloro-N-methyl-N-lysylglycin anilide), as adjunct antidotes in the treatment of organophosphorus intoxication in the guinea-pig. Journal of pharmacy and pharmacology, 42(4), 247-251.
- Wermuth, C. G. (Ed.). (2011). The practice of medicinal chemistry. Academic Press.
- Koike, M., Norikura, R., & Sugeno, K. (1986). Intestinal activation of a new sleep inducer 450191-S, a 1 H-1, 2, 4-triazolyl benzophenone derivative, in rats. Journal of pharmacobio-dynamics, 9(3), 315-320.
- Kangas, L., Kanto, J., & Syvälahti, E. (1977). Plasma nitrazepam concentrations after an acute intake and their correlation to sedation and serum growth hormone levels. Acta Pharmacologica et Toxicologica, 41(1), 65-73.
- Wildmann, J. H. W. U. K. R., Möhler, H., Vetter, W., Ranalder, U., Schmidt, K., & Maurer, R. (1987). Diazepam and N-desmethyldiazepam are found in rat brain and adrenal and may be of plant origin. Journal of neural transmission, 70(3), 383-398.
- Unseld, E., & Klotz, U. (1989). Benzodiazepines: are they of natural origin?. Pharmaceutical research, 6(1), 1-3.
- Preedy, V. R. (Ed.). (2016). Neuropathology of drug addictions and substance misuse volume 1: foundations of understanding, tobacco, alcohol, cannabinoids and opioids. Academic Press.
- Huang, Q., Zhang, W. J., Liu, R. Y., McKernan, R. M., & Cook, J. M. (1996). Benzo-fused benzodiazepines employed as topological probes for the study of benzodiazepine receptor subtypes. Medicinal Chemistry Research, 6(6), 384-391.
- Catalani, V., Botha, M., Corkery, J. M., Guirguis, A., Vento, A., Scherbaum, N., & Schifano, F. (2021). The psychonauts’ benzodiazepines; quantitative structure-activity relationship (Qsar) analysis and docking prediction of their biological activity. Pharmaceuticals, 14(8), 720.
- Greenblatt, D. J., Locniskar, A., Ochs, H. R., & Lauven, P. M. (1981). Automated gas chromatography for studies of midazolam pharmacokinetics. Anesthesiology, 55(2), 176-179.
- Zin’kovskii, V. G. (1976). Analysis of the structure of the components of the convulsive action of corazole following administration of sulazepam and its metabolites to mice. Biulleten’Eksperimental’noi Biologii i Meditsiny, 82(9), 1078-1081.
- Zhang, W., Koehler, K. F., Harris, B., Skolnick, P., & Cook, J. M. (1994). Synthesis of benzo-fused benzodiazepines employed as probes of the agonist pharmacophore of benzodiazepine receptors. Journal of medicinal chemistry, 37(6), 745-757.
- Oelschläger, H., Ellaithy, M. M., & Volke, J. (1988). Mechanismus der polarographischen Reduktion des Tranquilizers Uldazepam. Archiv der Pharmazie, 321(2), 69-72.
- Garred, P., Brygge, K., Sørensen, C. H., Madsen, H. O., Thiel, S., & Svejgaard, A. (1993). Mannan-binding protein—levels in plasma and upper-airways secretions and frequency of genotypes in children with recurrence of otitis media. Clinical & Experimental Immunology, 94(1), 99-104.
- Kumar, R., & Joshi, Y. C. (2008). Synthesis and antimicrobial, antifungal and anthelmintic activities of 3H-1, 5-benzodiazepine derivatives. Journal of the Serbian chemical society, 73(10), 937-943.
- Puodziunaite, B. D., Janciene, R., Kosychova, L., & Stumbreviciute, Z. (2000). On the synthetic way to novel peri-annelated imidazo [1, 5] benzodiazepinones as the potent non-nucleoside reverse transcriptase inhibitors. Arkivoc, 1(4), 512-522.
- Grossi, G., Di Braccio, M., Roma, G., Ballabeni, V., Tognolini, M., Calcina, F., & Barocelli, E. (2002). 1, 5-Benzodiazepines: Part XIII. Substituted 4H-[1, 2, 4] triazolo [4, 3-a][1, 5] benzodiazepin-5-amines and 4H-imidazo [1, 2-a][1, 5] benzodiazepin-5-amines as analgesic, anti-inflammatory and/or antipyretic agents with low acute toxicity. European journal of medicinal chemistry, 37(12), 933-944.
- Rubin, M. A., Albach, C. A., Berlese, D. B., Bonacorso, H. G., Bittencourt, S. R. T., Queiroz, C. M. T., … & Mello, C. F. (2000). Anxiolytic-like effects of 4-phenyl-2-trichloromethyl-3H-1, 5-benzodiazepine hydrogen sulfate in mice. Brazilian Journal of Medical and Biological Research, 33, 1069-1073.
- Cacchi, S., Fabrizi, G., Goggiamani, A., & Iazzetti, A. (2016). Construction of the 1, 5-Benzodiazepine skeleton from o-Phenylendiamine and propargylic alcohols via a domino gold-catalyzed Hydroamination/Cyclization process. Organic letters, 18(15), 3511-3513.
- Owen, R. T., & Tyrer, P. (1983). Benzodiazepine dependence. Drugs, 25(4), 385-398.
- Itil, T. M. (1976). Controlled clinical and quantitative EEG studies of triflubazam (ORF 8063) in patients with anxiety syndrome.
- Nicholson, A. N., Stone, B. M., & Clarke, C. H. (1977). Effect of the 1, 5‐benzodiazepines, clobazam and triflubazam, on sleep in man. British Journal of Clinical Pharmacology, 4(5), 567-572.
- Knafo, A., Israel, S., Darvasi, A., Bachner‐Melman, R., Uzefovsky, F., Cohen, L., … & Ebstein, R. P. (2008). Individual differences in allocation of funds in the dictator game associated with length of the arginine vasopressin 1a receptor RS3 promoter region and correlation between RS3 length and hippocampal mRNA. Genes, brain and behavior, 7(3), 266-275.
- Isbister, G. K., O’Regan, L., Sibbritt, D., & Whyte, I. M. (2004). Alprazolam is relatively more toxic than other benzodiazepines in overdose. British journal of clinical pharmacology, 58(1), 88-95.
- Teboul, E., & Chouinard, G. (1990). A guide to benzodiazepine selection. Part I: Pharmacological aspects. The Canadian Journal of Psychiatry, 35(8), 700-710.
- Sur, C., Fresu, L., Howell, O., McKernan, R. M., & Atack, J. R. (1999). Autoradiographic localization of α5 subunit-containing GABAA receptors in rat brain. Brain research, 822(1-2), 265-270.
- Iyer, S. V., Benavides, R. A., Chandra, D., Cook, J. M., Rallapalli, S., June, H. L., & Homanics, G. E. (2011). α4-containing GABAA receptors are required for antagonism of ethanol-induced motor incoordination and hypnosis by the imidazobenzodiazepine Ro15-4513. Frontiers in Pharmacology, 2, 18.
- Sharma, D., Dixit, A. B., Dey, S., Tripathi, M., Doddamani, R., Sharma, M. C., … & Banerjee, J. (2021). Increased levels of α4-containing GABAA receptors in focal cortical dysplasia: A possible cause of benzodiazepine resistance. Neurochemistry International, 148, 105084.
- Dias, R., Sheppard, W. F., Fradley, R. L., Garrett, E. M., Stanley, J. L., Tye, S. J., … & Reynolds, D. S. (2005). Evidence for a significant role of α3-containing GABAA receptors in mediating the anxiolytic effects of benzodiazepines. Journal of Neuroscience, 25(46), 10682-10688.
- Fritschy, J. M., & Mohler, H. (1995). GABAA‐receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. Journal of Comparative Neurology, 359(1), 154-194.
- Rudolph, U., & Möhler, H. (2014). GABAA receptor subtypes: Therapeutic potential in Down syndrome, affective disorders, schizophrenia, and autism. Annual review of pharmacology and toxicology, 54, 483.
- Rudolph, U., & Knoflach, F. (2011). Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nature reviews Drug discovery, 10(9), 685-697.
- Patočka, J., & Jakl, J. (2010). Biomedically relevant chemical constituents of Valeriana officinalis. Journal of applied biomedicine, 8(1), 11-18.
- Andreatini, R., & Leite, J. (1994). Effect of valepotriates on the behavior of rats in the elevated plus-maze during diazepam withdrawal. European journal of pharmacology, 260(2-3), 233-235.
- Nathan, P. J., Lu, K., Gray, M., & Oliver, C. (2006). The neuropharmacology of L-theanine (N-ethyl-L-glutamine) a possible neuroprotective and cognitive enhancing agent. Journal of Herbal Pharmacotherapy, 6(2), 21-30.