MDPV: Exploring One of the Most Powerful Stimulants on Earth
One of the three first-generation substituted cathinones, MDPV remains one of the most popular compounds in its class (and possibly the most potent).
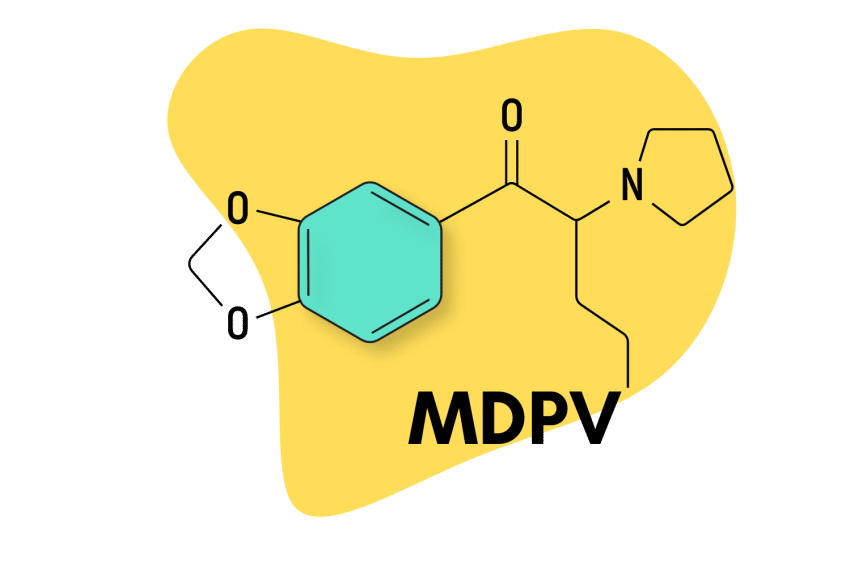
Developed in the 1960s by a team to Boehringer Ingelheim, MDPV (methylenedioxypyrovalerone) is a psychostimulant of the substituted cathinone family (which itself is a sub-group of the amphetamine class of drugs).
Members of the cathinone subtype are based on the chemical structure of basic cathinone — a naturally-occurring alkaloid from the khat plant (Catha edulis).
MDPV carries the signature trademarks of its class: a strong emphasis on stimulant properties while still retaining some of the hallucinogenic and empathogenic qualities of a drug like MDMA and incredible potency.
In fact, along with α-PPP and α-PVP, MDPV is one of the most powerful drugs in its class.
Cathiones are also known to be highly addictive, and MDPV is no exception.
Starting around 2004, MDPV and other cathinones became available in the United States, often sold at gas stations under the name of “bath salts,” until a public outcry led to their banning in 2011.
This episode has led to a warped public perception of substituted cathinone, with many people believing this drug type is sure to induce mania and bouts of rage in its users. Such cases have been documented but are usually the result of polydrug abuse.
In reality, substituted cathinones are quite dangerous, but, as usual, the public perception is too sensationalist.
MDPV Specs
| Chemical Name | Methylenedioxypyrovalerone |
| IUPAC Name | 1-(1,3-benzodioxol-5-yl)-2-pyrrolidin-1-ylpentan-1-one |
| Other names | Bath salts, Monkey dust |
| Status | Research Chemical / Schedule I on the Controlled Substances Act |
| Duration of Effects | 3 to 7 hours |
| Estimated Threshold Dose | 2 mg |
| Common Dose | 5 to 15 mg |
| PubChem ID | 20111961 |
| CAS | 687603-66-3 |
Metabolism
MDPV’s metabolic process is mediated by the CYP450 system, specifically the 2D6, SC19, and 1A2 enzymes [1], and occurs in the liver, where the transformation method is COMT phase 1 metabolism. It is transformed into methylcatechol and pyrrolidine, which in turn are glucuronated (uridine 5′-diphospho-glucuronosyl-transferase), allowing the products to be excreted by the kidneys, with only a small fraction of the metabolites being excreted into the stools. No free pyrrolidine is detected in the urine [2].

Molecularly, this is seen as demethylenation of methylenedioxypyrovalerone, followed by methylation of the aromatic ring via catechol-O-methyl transferase. Hydroxylation of both the aromatic ring and side chain then takes place, followed by oxidation of the pyrrolidine ring to the corresponding lactam, with subsequent detachment and ring opening to the corresponding carboxylic acid.
4-OH-3-MeO-PV is the major metabolite found in urine samples from rats and humans exposed to MDPV administration.
Duration of Effects
According to the DEA, MDPV’s effects have a duration of three to four hours, with the after-effects lasting another three to four hours. Adverse effects like tachycardia, hypertension, sweating, and vasoconstriction usually last up to six to eight hours after administration.
How Does MDPV Work?
Substituted cathinones like MDPV work by affecting the neuropharmacology of neuronal transporters like dopamine and serotonin through three basic mechanisms:
- Some block the reuptake of neurotransmitters
- Some block the clearance of neurotransmitters
- Some reverse the polarity of neurotransmitter transporters
Inside the body, the reality is, of course, far more complex: typically, psychostimulant compounds will utilize more than one mechanism, and differences in receptor affinities also come into play. Simply stated, the more in-depth we go into the neuropharmacology of these products, the more differences we can find, but that being said, there is still a high degree of similarity between them.

Psychostimulant amphetamines utilize a combination of different methods, but they all achieve the same goal — an increased level of active neurotransmitters within the brain.
Primarily, MDPV is classified as a norepinephrine–dopamine reuptake inhibitor (NDRI). Research has found that its activity at the dopamine transporter is six times stronger than at the norepinephrine transporter, and it is virtually inactive at the serotonin transporter [3]. This would make MDPV almost an exclusively dopaminergic drug, which accounts for its marked emphasis on stimulant properties.
In vivo, studies have revealed that, when compared to the prototypical uptake inhibitor cocaine, MDPV is 50 times more potent as an inhibitor of dopamine (DAT), 10 times more potent at norepinephrine receptors (NET) and tenfold less potent at serotonin receptors (SERT). It was also found that unlink other compounds in its class, like 4-MMC and methylone, MDPV does not act as a substrate for monoamine transporters, meaning that it does not have the capability to stimulate the release of neurotransmitter molecules from presynaptic neurons – a secondary mechanism of action present in many psychostimulants [3].
These findings for MDPV’s pharmacology are quite interesting as it appears to have basically no secondary mechanisms acting as a “pure” reuptake inhibitor. What’s more, MDPV’s affinity for serotonin is not significant, while action at secondary sites like adrenergic receptors is non-existent. In this sense, MDPV is also a purely dopaminergic drug.
MDPV Pharmacokinetics
Given the importance of pharmacokinetics (PK) in the forensic detention and evaluation of NPS, there is a good amount of research on the subject, although mostly in rat studies.
Like other drugs in its class, MDPV’s PK values were rapid. In rats, maximal plasma concentrations occurred within 15 to 20 minutes after injection and decreased quickly thereafter. Upon injection of 2 mg of MDPV, the plasma Cmax for the drug is 271 μg/L (~1 μM), and the elimination half-life is about 80 minutes. Plasma concentrations for the metabolites, 3,4-catechol-PV and 4-OH-3-MeO-PV, increased at a much slower rate, with maximum concentrations being reached in three to four hours post-injection [4].
It was also found that plasma MDPV concentrations display linear dose-proportional kinetics. This was surprising for researchers, as similar drugs like MDMA, through sustained inhibition of CYP enzymes, have been shown to accumulate in humans in a more exponential rather than linear fashion [4].
When experimenting with different methods of administration on rats, researchers recorded different PK values. This gives scientific backing to the recreational behavior often seen in relation to psychostimulant use, in which users will utilize a variety of methods, like snorting and “bombing,” in order to achieve both short and long-lasting effects. However, in the methods utilized by researchers, PK values always remained relatively fast, suggesting the efficacy of utilizing different methods is limited.
What Are the Effects of MDPV?
Substituted cathinones are well-known stimulants. Users often describe them as being similar to cocaine but with a little pinch of MDMA thrown in there.
For a drug like MDPV, the scientific literature suggests that action at the serotonin receptor – linked to empathogenic and psychedelic properties – is so small that there should be little comparison in terms of the subjective feeling when compared to the stimulant properties. However, user reports consistently express that MDMA-like qualities are present, albeit to a much lower extent.

Posts found on online drug forums, often the only type of human-based information that can be found on NPS, mention the following cognitive and physical effects of MDPV:
- Compulsive redosing
- Confusion (prominent at higher doses and long bouts of wakefulness)
- Delusions
- Diarrhea
- Empathy, love, and sociability
- Increased heart rate
- Nausea (relatively uncommon)
- Psychosis (high doses of MDPV have been linked with psychosis at a more frequent rate than other stimulants)
- Thought disorganization (manifests at higher doses)
- Thought organization (mainly observed at low doses)
- Time distortion
- Wakefulness
After effects of MDPV are mainly composed of:
- Anxiety
- Cognitive fatigue
- Depression
- Irritability
- Motivation suppression
- Thought deceleration
- Wakefulness
When it comes to scientific studies, rat data shows how all synthetic cathinones are known to stimulate locomotor activity and induce increased heart rate and blood pressure.
In a study by Marusich et al., it was shown that, in terms of ambulation, MDPV is much more potent than 4-MMC and methylone [5]. Other studies have concluded that MDPV is about ten times more potent than cocaine as a locomotor stimulant in rats [6].
As can be surmised, MDPV is an extremely potent stimulant, having the most psychomotor activity in a subtype that’s already known for its powerful stimulant effects.
Researchers have suggested that MDPV’s lack of serotonin activation is the reason for its potent stimulant properties. Although the findings are still muddy, many believe that serotonin increases play an important role in counteracting the heavy stimulant effects of dopamine and norepinephrine modulation. In this sense, it seems that MDPV’s heavy dopamine stimulation is not meaningfully counterbalanced by serotonin, and this is what gives rise to its stimulant-heavy profile.
Is MDPV Safe? Risks & Side Effects
MDPV is classified in the Controlled Substances Act (CSA) as a Schedule I substance, meaning it has no medicinal value and presents a high potential for abuse and dependence. In general, MDPV seems to present largely the same danger as other compounds in its class, but there are some individual characteristics that make its risk profile unique.
MDPV is one of the most potent compounds in its class, which has the immediate effect of making it more dangerous. Its elevated potency entails a narrow dosage window, which means that taking just a little bit too much can lead to psychosis, heart failure, stroke, or permanent brain damage.

MDPV also seems to be the more stimulant-heavy of the synthetic cathinones, which likely has the effect of increasing the prevalence of stimulant-related adverse effects. In this sense, adverse effects related to overstimulation, like hyperthermia and tachycardia, are far more dangerous than those related to psychedelia, like nausea, confusion, or having a “bad trip.”
However, certain factors suggest that, in other regards, MDPV might be safer when compared to similar compounds.
For instance, research has found that MDPV is not a compound that acts as a transporter substrate – these are often called “releasers” as they induce non-exocytotic transporter-mediated neurotransmitter release from neurons. As such, it’s theorized that MDPV does not cause the negative effects that have been linked to this mechanism: mainly the production of intracellular deficits in monoamine neurons, such as inhibition of neurotransmitter synthesis and disruption of vesicular storage, leading to long-term neurotransmitter depletion [6].
PK analysis of MDPV also suggests that it’s more prone to linear accumulation rather than exponential. In the context of repeated administration, a use habit strongly associated with psychostimulants would make MDPV safer than another compound like MDMA, which has been shown to accumulate non-linearly as a result of stronger CYP inhibition.
Side Effects of MDPV
The side effects of psychostimulant use have been fairly studied. When it comes to MDPV, users report a high degree of similarity with the adverse effects related to cocaine use
Studies on MDPV have found the following adverse effects [7]:
- Agitation
- Anxiety
- Arrhythmia
- Cardiac arrest
- Cardiotoxic effects (cardiomyopathy, acute coronary syndrome, etc.)
- Cerebral vasoconstriction
- Coma
- Confusion
- Dehydration
- Disorganized thoughts
- Disturbed sleep patterns
- Hypertension
- Hyperthermia
- Involuntary body movements
- Loss of appetite
- Paranoia
- Psychotic symptoms
- Residual depression
- Restlessness
- Suicidal ideation
- Sweating
- Tachycardia
- Violent behavior
After surveying user reports on drug forums, it appears that episodes of psychosis are particularly prevalent when using MDPV.
Withdrawal & Dependence
Compounds in the synthetic cathinone class have been proven to be highly addictive [8]. However, it’s important to put these findings into context.
Pharmacologic evidence for physical addiction is still somewhat lacking when it comes to psychostimulant drugs. The addiction mechanism, on the other hand, appears to come as a result of the activation of reward circuitry in the brain, which is a behavioral system. Withdrawal symptoms have been documented, though, which suggests the development of physical dependence.

In any case, due to their recreational nature and their rapid pharmacokinetics, the abuse liability of psychostimulant drugs is high, as the formation of addictive habits is likely.
Research suggests the abuse potential for MDPV is especially high.
In rat self-administration studies, it was found that MDPV self-administration habits formed much more rapidly when compared to methylone. Additionally, the number of infusions per session is significantly greater for MDPV. As a consequence of these results, researchers speculate that the lack of serotonergic action in MDPV use leads to higher addictive potential [6].
Harm Reduction: MDPV
With the exception of some promising new developments with regard to their therapeutic potential, psychostimulants are used in a context that’s almost exclusively recreational. Harm reduction techniques are thus incredibly important, and it’s crucial for all psychostimulant users to be familiar with them.
We’ll now break down some of the most important techniques that will help keep you safe when using psychostimulants:
1. Be Aware of the Potency
A general truism when using any type of drug is “more potency = more danger.” MDPV is exceptionally potent and therefore carries a higher risk of overdose-related side effects.
2. Always Test Your Drugs
The use of illegal drugs will always be more dangerous because of the ever-present threat of adulteration. To hedge against this risk, we always recommend users test their substances using a reagent test kit.
These kits cost less than $40 and provide enough for hundreds of tests. Certain countries have also allowed harm-reduction groups to operate testing centers at events where drug use is common such as festivals or raves. If you go to an event and see one of these centers, we suggest you make use of it.

3. Avoid Polydrug Use
The use of more than one drug at a time is always a bad idea. Drug combinations are complex things, and when more than one drug is introduced into the system, the resulting effects are virtually always negative. Some combinations are a lot more dangerous than others, though. For example, the concomitant use of central nervous system depressants is responsible for the majority of drug-related fatalities in the United States.
In the case of psychostimulants, their combination with depressants can mask the depressive effect, often leading users to ingest acute doses of depressant compounds.
MDPV Dosage
Information on MDPV dosage is based on subjective self-assessment reports, so they should always be taken with a grain of salt. It’s highly recommended that users, especially first-time users, start with a low or threshold dose and then work their way up.
The common dosage values for MDPV can be found below:
| Threshold | 2 mg |
| Light | 4 – 8 mg |
| Common | 8 – 14 mg |
| Strong | 14 – 25 mg |
| Heavy | 25+ mg |
MDPV FAQs
1. How does MDPV’s structure relate to its pharmacology? How doe MDPV compare to other compounds in its class?
In an informative structure-activity study, Kolanos et al. found that, in the MDPV molecule, the bulky pyrrolidine ring and the flexible α-carbon chain are critical attributes for potent uptake inhibition at DAT, whereas the 3,4-methylenedioxy ring moiety is of little consequence in this regard [6].
2. What are the preferred routes of administration for MDPV?
MDPV is primarily used orally, by snorting (insufflation), and by smoking. Less common routes of administration include intravenous injection, buccal, sublingual, and rectal application [7].
3. Are MDPV’s metabolites pharmacologically active?
Research has found that locomotor activity goes down in proportion to the formation of MDPV metabolites. This suggests that the parent compound is the major factor contributing to the locomotor stimulant effects of the drug.
References
- Kalapos M. P. (2011). 3,4-methylene-dioxy-pyrovalerone (MDPV) epidemic? Orvosi Hetilap, 152(50), 2010–2019. https://doi.org/10.1556/OH.2011.29259
- Strano-Rossi, S., Cadwallader, A. B., De La Torre, X., & Botrè, F. (2010). Toxicological determination and in vitro metabolism of the designer drug methylenedioxypyrovalerone (MPDV) by gas chromatography/mass spectrometry and liquid chromatography/quadrupole time-of-flight mass spectrometry. Rapid Communications in Mass Spectrometry, 24(18), 2706–2714. https://doi.org/10.1002/rcm.4692
- Baumann, M. H., Partilla, J. S., Lehner, K. R., Thorndike, E. B., Hoffman, A. F., Holy, M., Rothman, R. B., Goldberg, S. R., Lupica, C. R., Sitte, H. H., Brandt, S. D., Tella, S. R., Cozzi, N. V., & Schindler, C. W. (2013). Powerful Cocaine-Like Actions of 3,4-Methylenedioxypyrovalerone (MDPV), a Principal Constituent of Psychoactive ‘Bath Salts’ Products. Neuropsychopharmacology, 38(4), 552–562. https://doi.org/10.1038/npp.2012.204
- Anizan, S., Concheiro, M., Lehner, K. R., Bukhari, M. O., Suzuki, M., Rice, K. C., Baumann, M. H., & Huestis, M. A. (2016). Linear pharmacokinetics of 3,4-methylenedioxypyrovalerone (MDPV) and its metabolites in the rat: Relationship to pharmacodynamic effects: MDPV PK and PD in Rats. Addiction Biology, 21(2), 339–347. https://doi.org/10.1111/adb.12201
- Marusich, J. A., Grant, K. R., Blough, B. E., & Wiley, J. L. (2012). Effects of synthetic cathinones contained in “bath salts” on motor behavior and a functional observational battery in mice. NeuroToxicology, 33(5), 1305–1313. https://doi.org/10.1016/j.neuro.2012.08.003
- Baumann, M. H., Bukhari, M. O., Lehner, K. R., Anizan, S., Rice, K. C., Concheiro, M., & Huestis, M. A. (2016). Neuropharmacology of 3,4-Methylenedioxypyrovalerone (MDPV), Its Metabolites, and Related Analogs. In M. H. Baumann, R. A. Glennon, & J. L. Wiley (Eds.), Neuropharmacology of New Psychoactive Substances (NPS) (Vol. 32, pp. 93–117). Springer International Publishing. https://doi.org/10.1007/7854_2016_53
- Karila, L., Lafaye, G., Scocard, A., Cottencin, O., & Benyamina, A. (2018). MDPV and α-PVP use in humans: The twisted sisters. Neuropharmacology, 134, 65–72. https://doi.org/10.1016/j.neuropharm.2017.10.007
- Baumann, M. H. (2014). Awash in a sea of “bath salts”: Implications for biomedical research and public health. Addiction (Abingdon, England), 109(10), 1577–1579. https://doi.org/10.1111/add.12601