2C Psychedelics
Not quite MDMA, not quite LSD, but a little bit of both.
Most people have heard of mescaline, LSD, and MDMA — but only serious psychonauts are familiar with the 2C substances.
The most popular members of the group are 2C-B and 2C-I — but there are dozens of other compounds that share similar effects.
Learning about the 2Cs is one of the best ways to understand psychedelic chemistry. The legendary chemist, Alexander Shulgin, created the group by systematically modifying the base molecule for mescaline by adding slightly different functional groups to one side of the molecule and then testing their effects.
Some of these creations proved to be formidable psychedelics and empathogens; others were completely inactive.
Here’s a crash course on the entire 2C family of psychedelics.
What Are 2C Psychedelics?
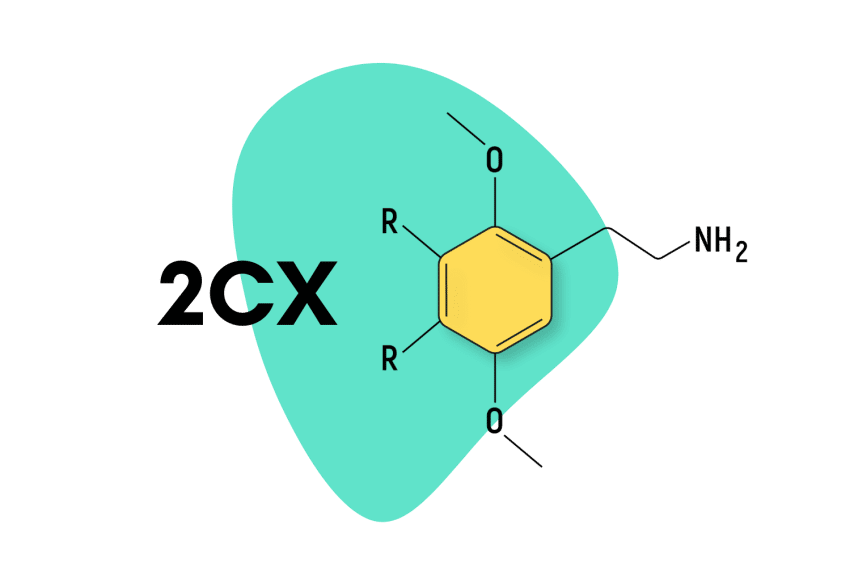
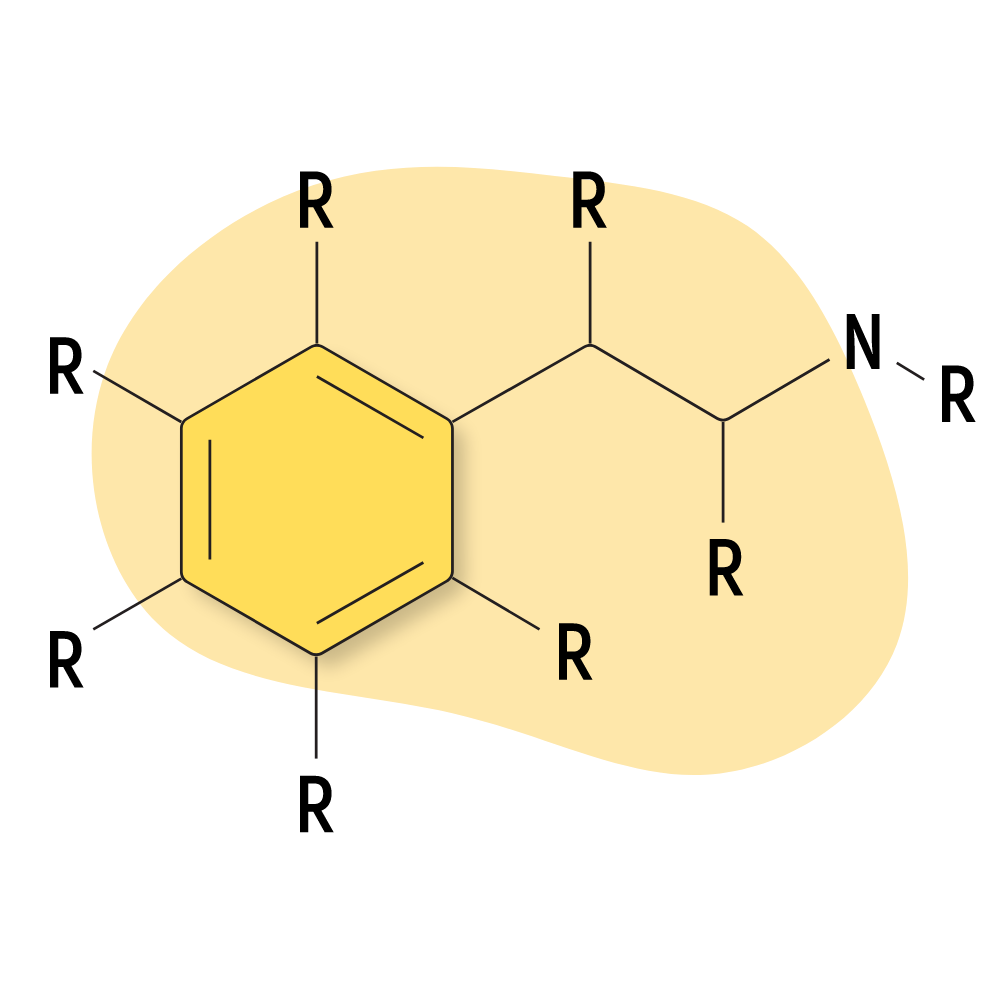
The 2CX family is derived from mescaline. They’re members of the greater phenethylamine class of psychedelics and are characterized by the presence of 2 carbon atoms located between a benzene ring and an amino group (hence the name “2C,” which implies “2 carbons”).
All 2C compounds contain 2 methoxy groups at the 2 and 5 positions of a central benzene ring. This leaves two additional regions for other functional groups to be attached. Shulgin would attach all kinds of different molecules to the R3 and R4 positions. In general, lipophilic functional groups at the R4 position produce the strongest and longest-lasting psychedelic effects.
2C compounds range from being inactive to powerfully empathogenic and psychedelic.
After MDMA was banned in the 1980s, 2C compounds became a popular “legal” alternative. That is until they, too, were banned in 1995.
Today, 2C substances remain popular in niche circles but have failed to capture a mainstream audience the way LSD or MDMA have. They’re often taken at raves or music festivals for their euphoric and hallucinogenic yet coherent effect profiles.
List Of The Most Popular 2C-X Psychedelics
There are well over 70 known members of the 2C-X family of psychedelics — most of them have not been adequately studied. Even 2C-B, which is by far the most popular, has never been examined in a clinical setting.
With that aside, here are some of the most common members of the 2C family.
| Image | Name | Discovered By | IUPAC Name |
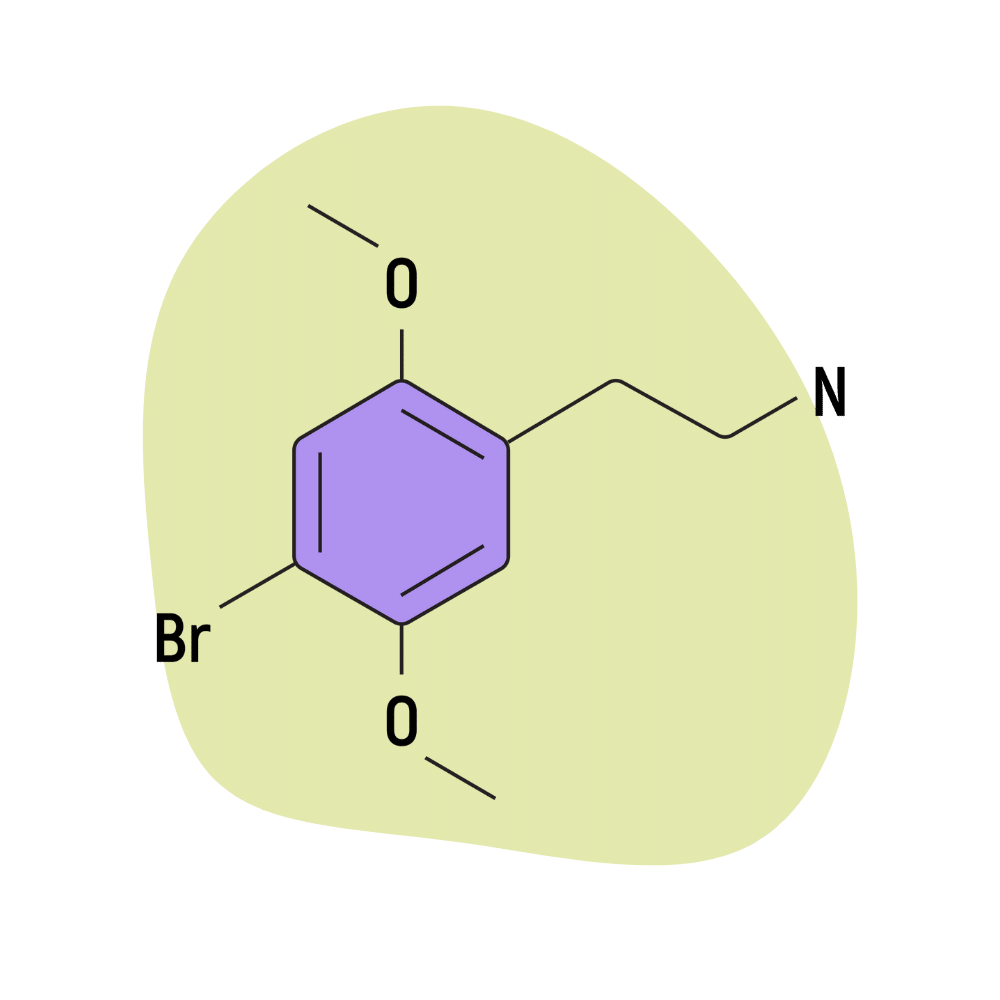
| 2C-B | Alexander Shulgin | 2,5-dimethoxy-4-bromophenethylamine |
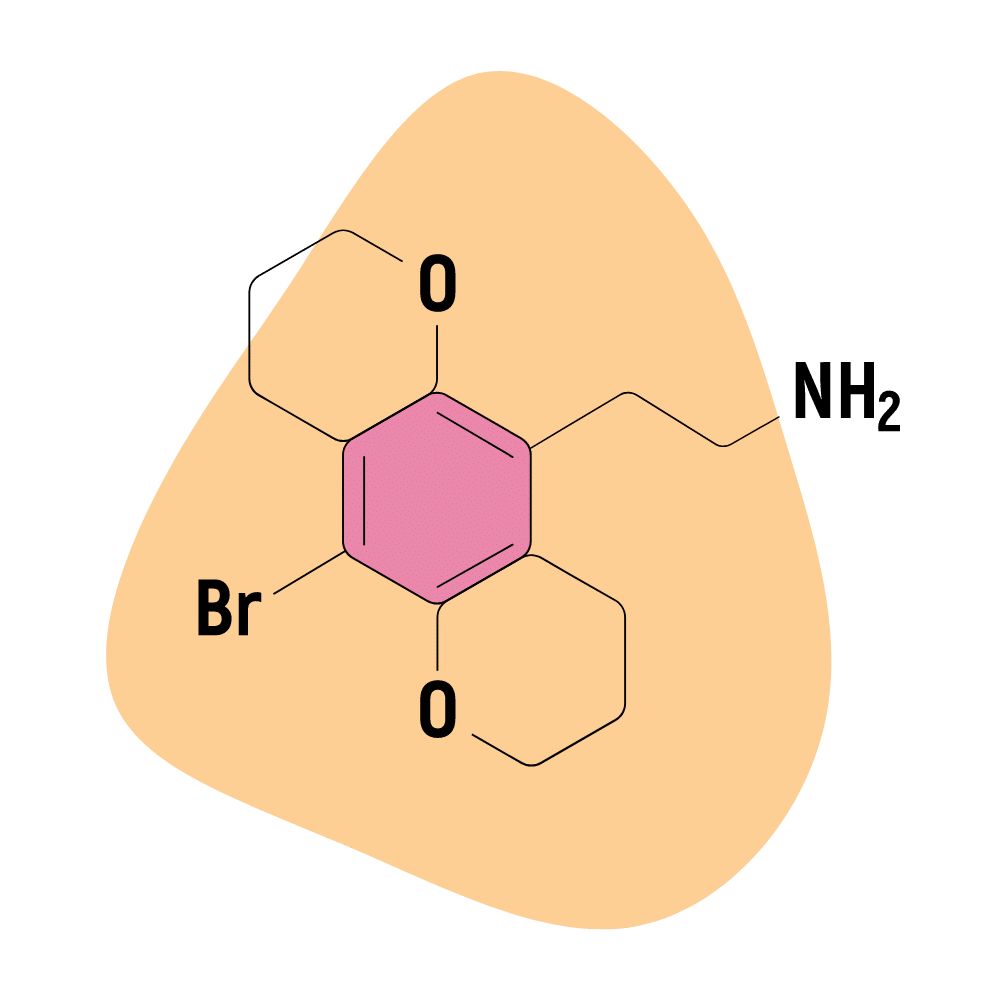
| 2C-B-BUTTERFLY | Michael S. Whiteside & Aaron Monte | 2-(5-bromo-2,3,4,7,8,9-hexahydropyrano[2,3-g]chromen-10-yl)ethanamine |
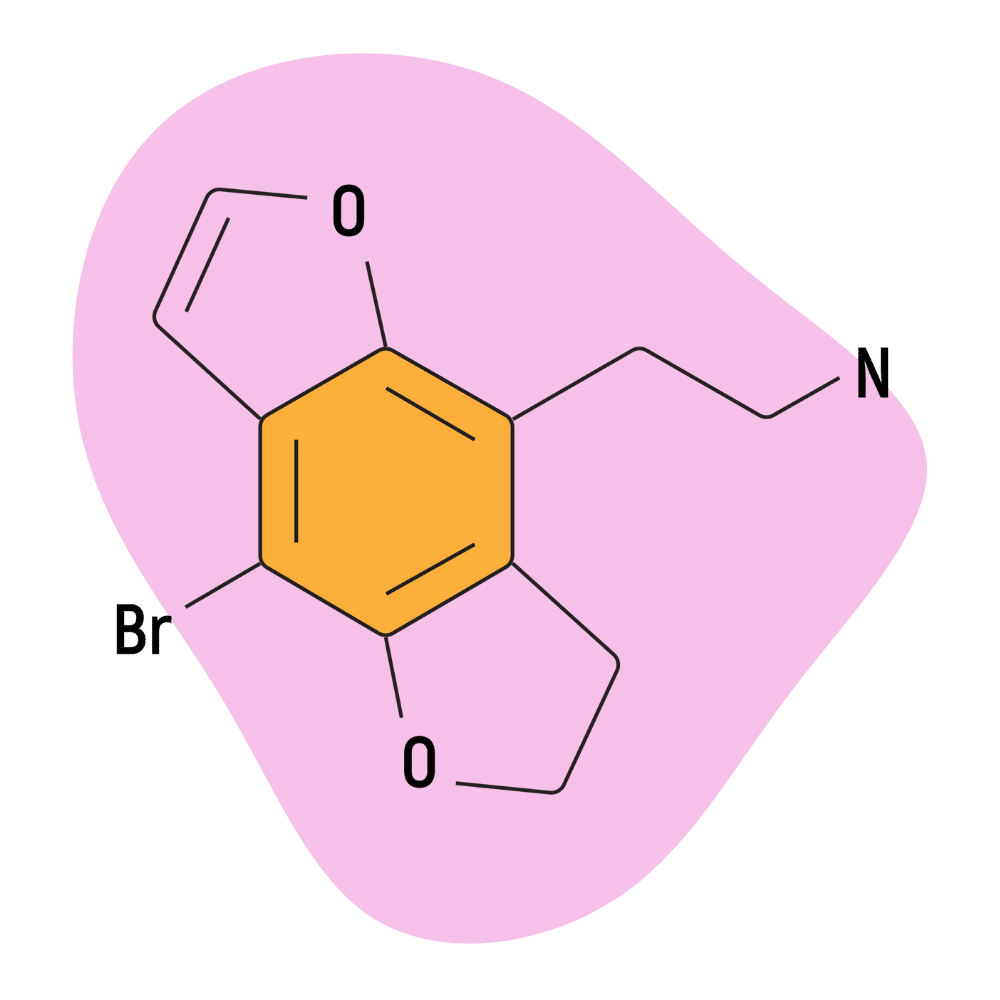
| 2C-B-DRAGONFLY | Aaron P. Monte | 2-(4-bromofuro[2,3-f][1]benzofuran-8-yl)ethanamine |
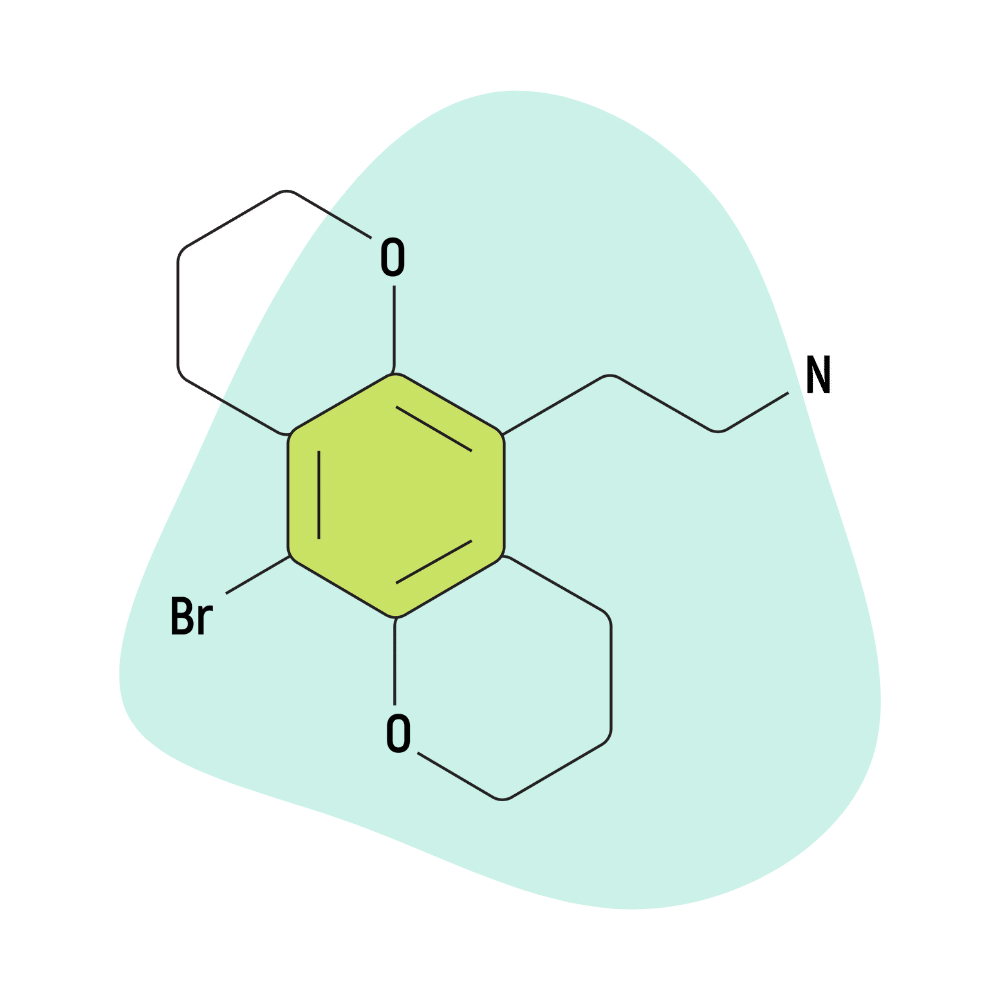
| 2C-B-FLY | Aaron P. Monte | 8-bromo-2,3,6,7-benzo-dihydro-difuran-ethylamine |
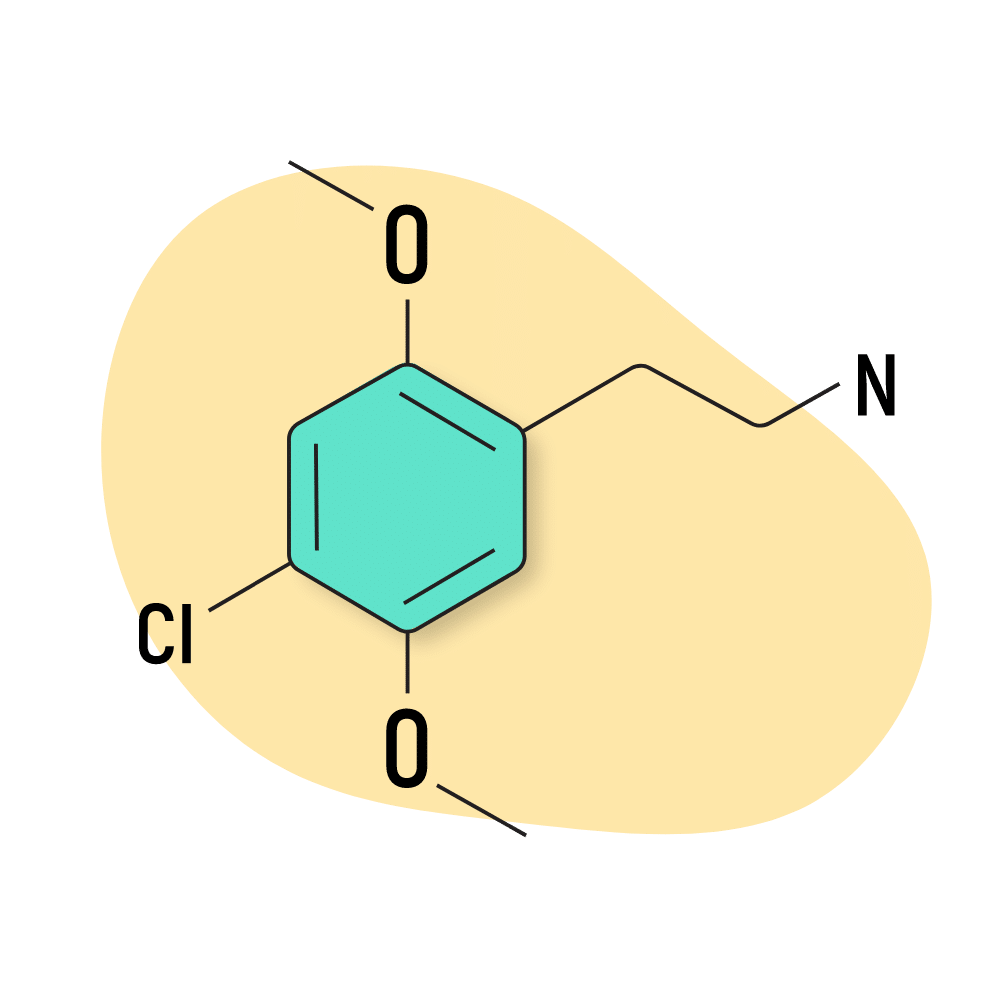
| 2C-C | Alexander Shulgin | 2,5-dimethoxy-4-chlorophenethylamine |
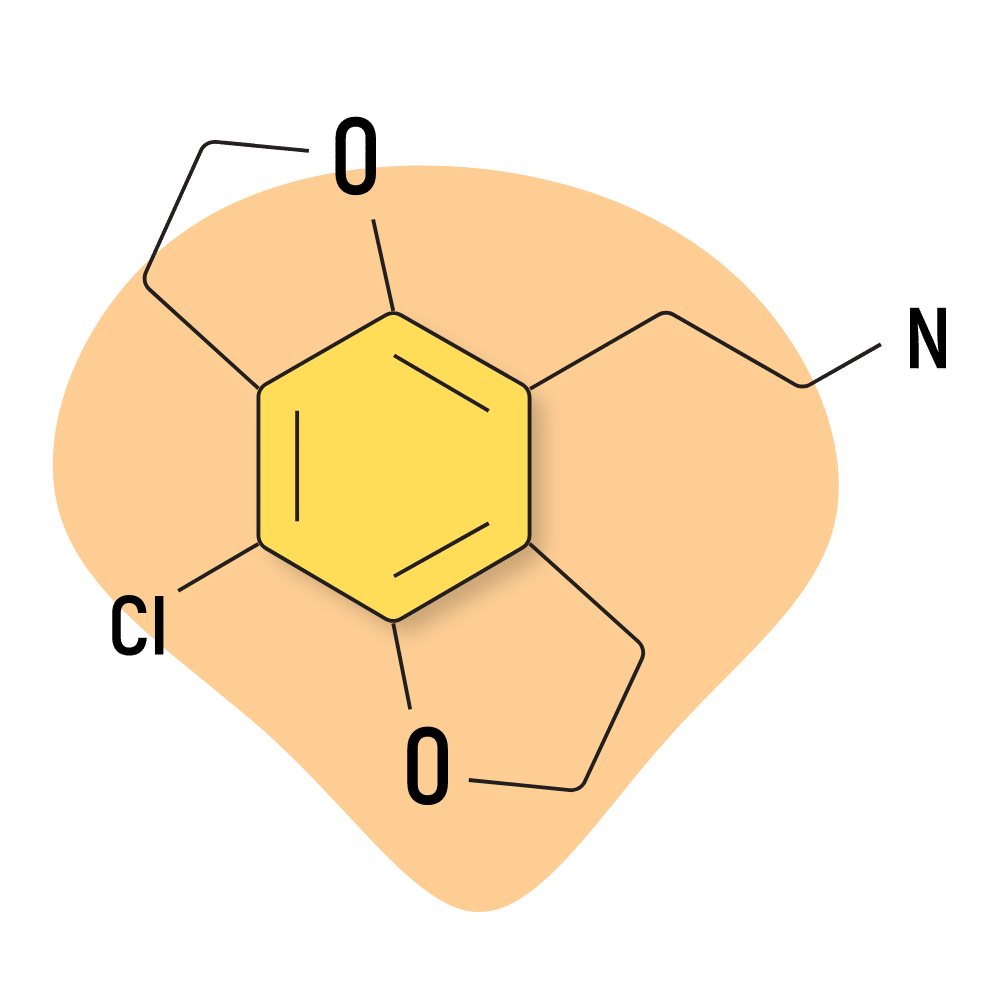
| 2C-C-FLY | Unknown | 2-(8-Methyl-2,3,6,7-tetrahydro-1,5-dioxa-s-indacen-4-yl)ethylamine |
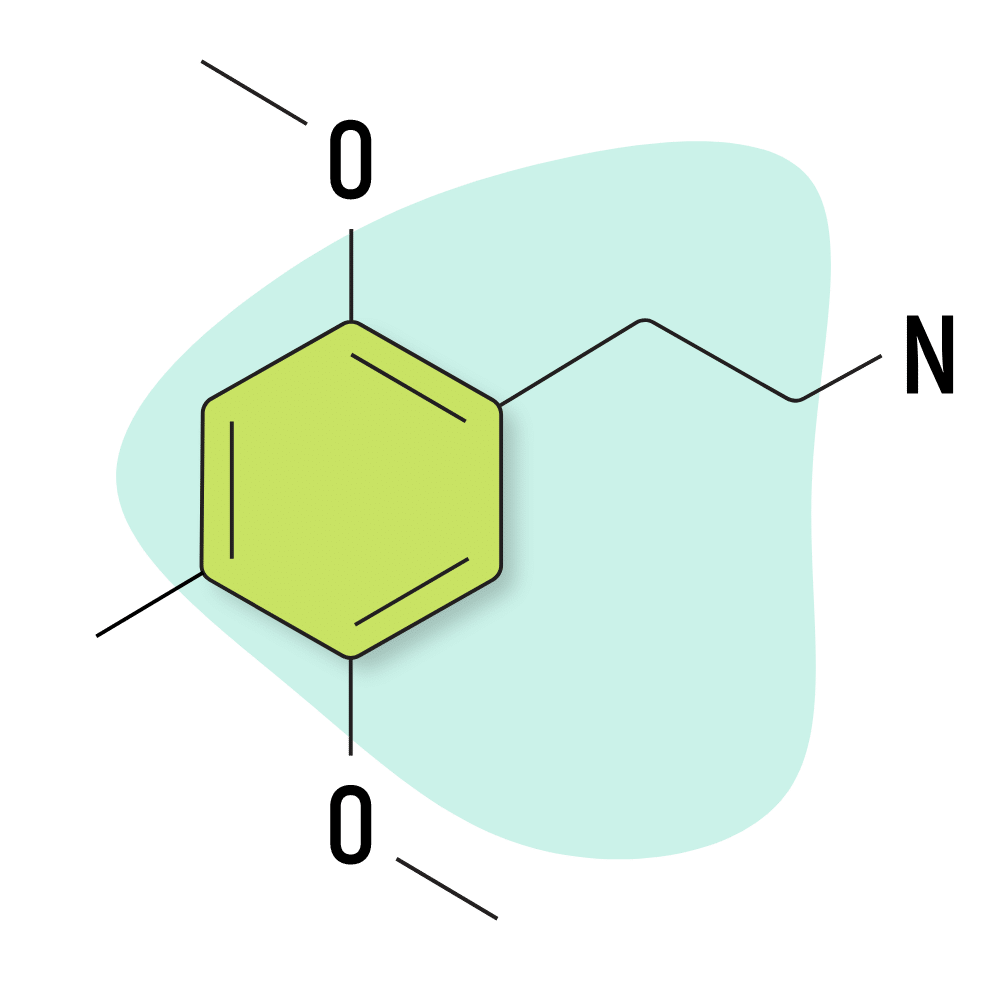
| 2C-D | Texas Research Institute of Mental Sciences | 2,5-dimethoxy-4-methylphenethylamine |
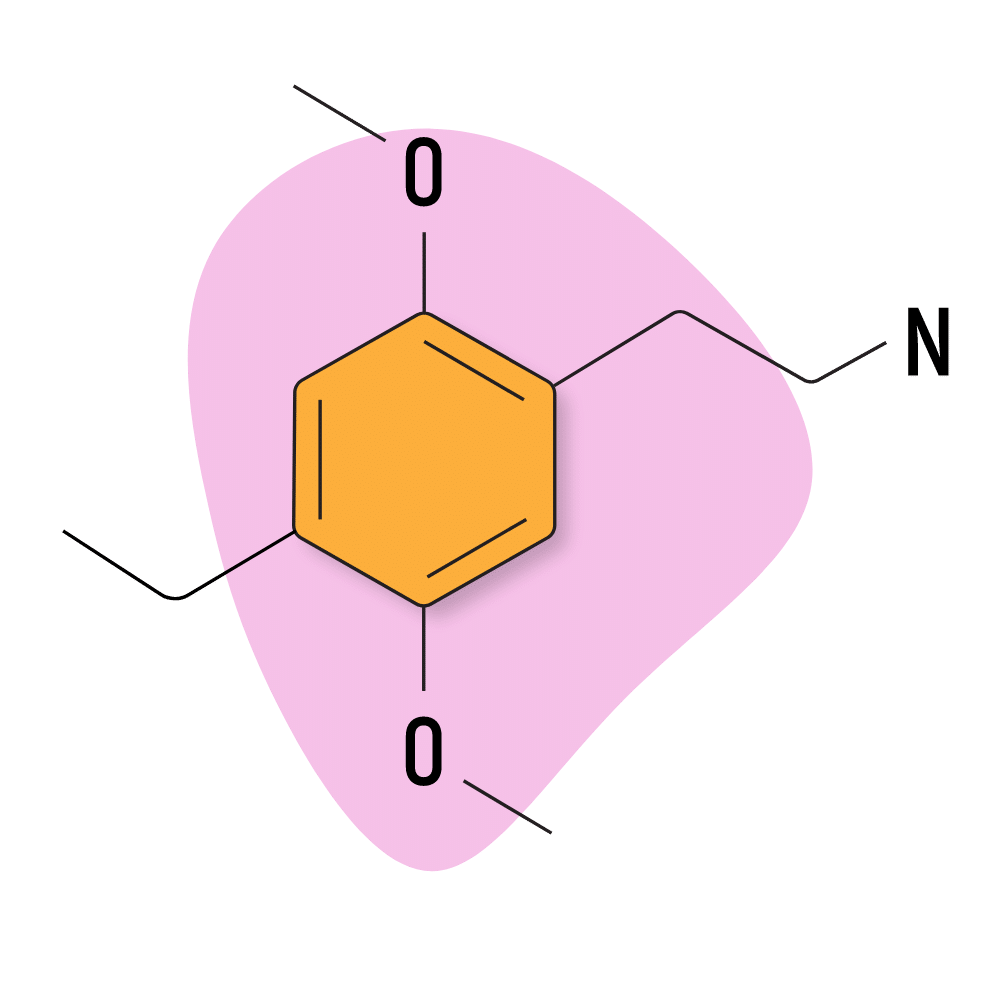
| 2C-E | Alexander Shulgin | 2,5-dimethoxy-4-ethylphenethylamine |
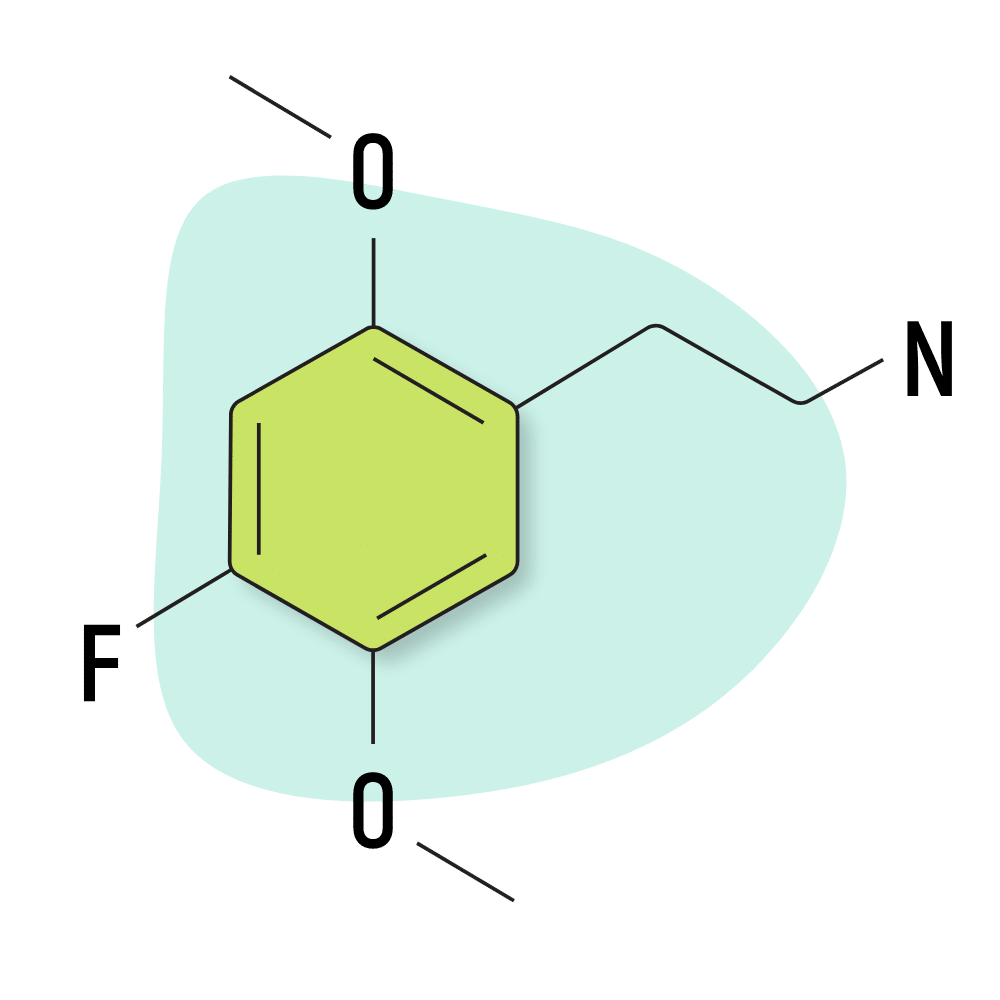
| 2C-F | Alexander Shulgin | 2,5-dimethoxy-4-fluorophenethylamine |
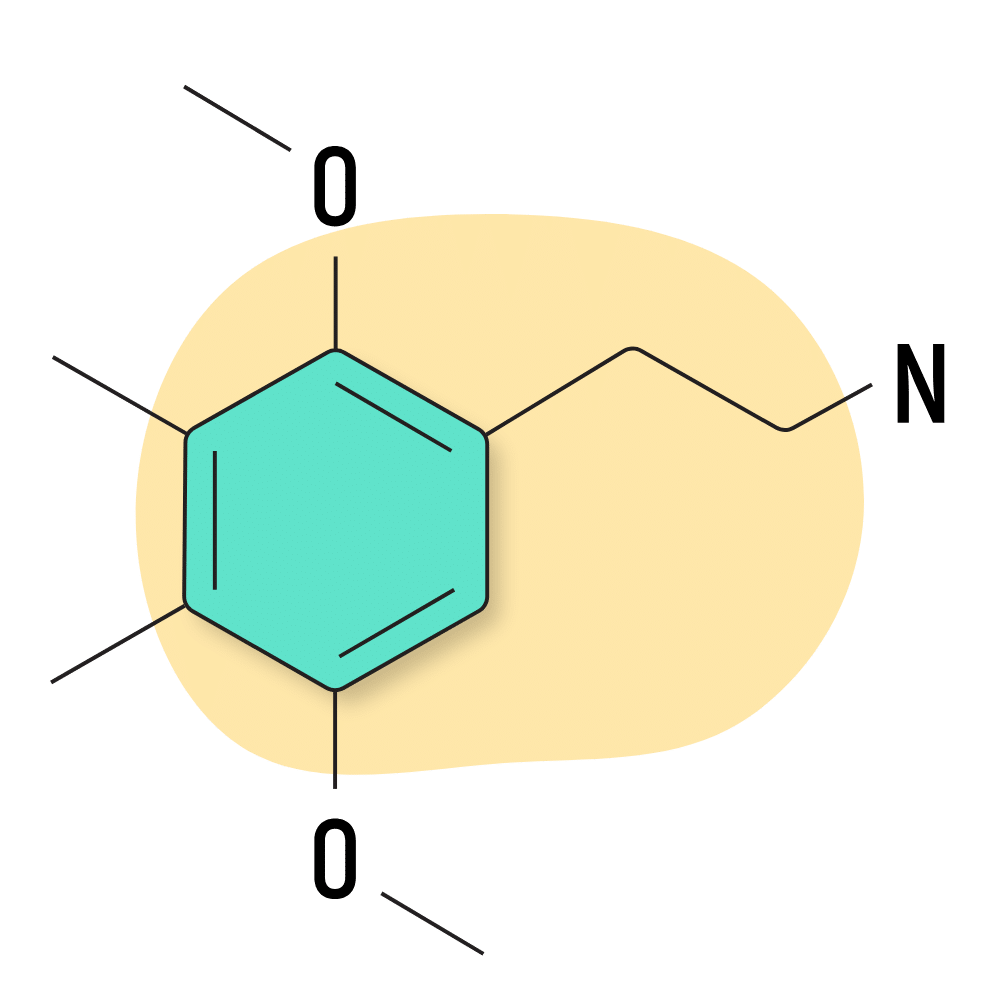
| 2C-G | Alexander Shulgin | 2-(2,5-Dimethoxy-3,4-dimethylphenyl)ethan-1-amine |
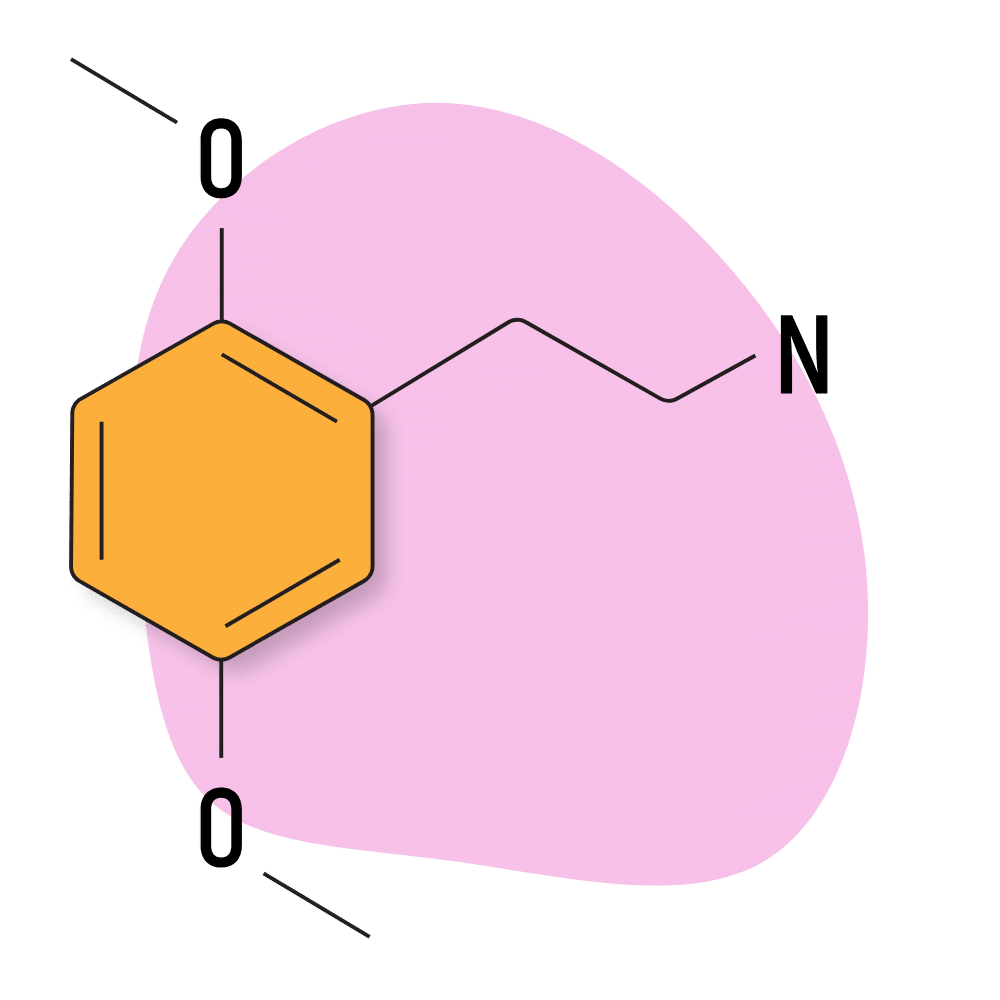
| 2C-H | Johannes S. Buck | 2,5-dimethoxyphenethylamine |
| 2C-I | Alexander Shulgin | 2,5-dimethoxy-4-iodophenethylamine |
| 2C-iP | Dmitri Ger | 4-Isopropyl-2,5-dimethoxyphenethylamine |
| 2C-N | Alexander Shulgin | 2,5-dimethoxy-4-nitrophenethylamine |
| 2C-O | Jansen | 2,4,5-trimethoxyphenethylamine |
| 2C-P | Alexander Shulgin | 2,5-dimethoxy-4-propylphenethylamine |
| 2C-T-2 | Alexander Shulgin | 2,5-dimethoxy-4-ethylthio-phenethylamine |
| 2C-T-7 | Alexander Shulgin | 2,5-dimethoxy-4-propylthio-phenethylamine |
| 2C-V | Daniel Trachsel | 2-(4-ethenyl-2,5-dimethoxyphenyl)ethanamine |
Simple 2C-X Compounds
Most members of the 2C-X group are considered “basic” in that they feature alterations to the R3 or R4 positions on the base molecule. More complicated alterations include the benzofurans and NBOMe compounds.
Many (but not all) basic 2C psychedelics are psychoactive and offer similar effects on cognition and sensory perception.
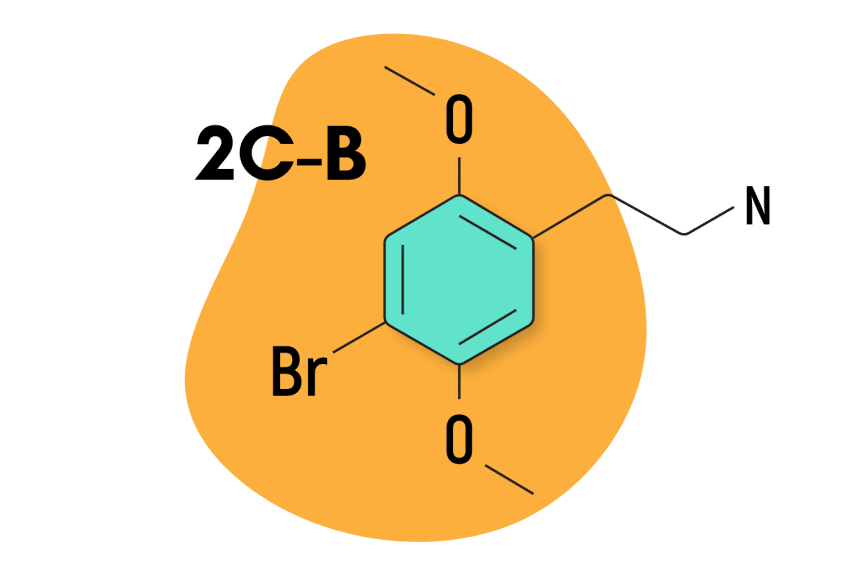
2C-B
2C-B is the most popular of Alexander Shulgin’s discoveries. It’s widely used in rave subculture for its blend of entactogen and psychedelic qualities. The best way to describe the effects of this compound is that it’s somewhere in between MDMA and LSD.
In the 80s, the German pharmaceutical company, Drittewelle, sold 2C-B under the name ‘Nexus’ in sex shops as a tool for boosting sexual connection and intimacy. 2C-B is still used for this purpose today.

2C-B Specs:
| Chemical Name | 4-bromo-2,5-dimethoxyphenethylamine |
| Status | Research Chemical 🧪 |
| Duration of Effects | 4–8 hours |
| Estimated Threshold Dose | 5 mg |
| Common Dose | 15–25 mg |
| PubChem ID | 98527 |
| CAS# | 66142-81-2 |
There are several “spin-offs” of 2C-B as well, including:
- 2C-B-FLY
- 2C-B-BUTTERFLY
- 2C-B-DRAGONFLY
- 2C-B-NBOME
- 2C-Bn
- 2C-Bu
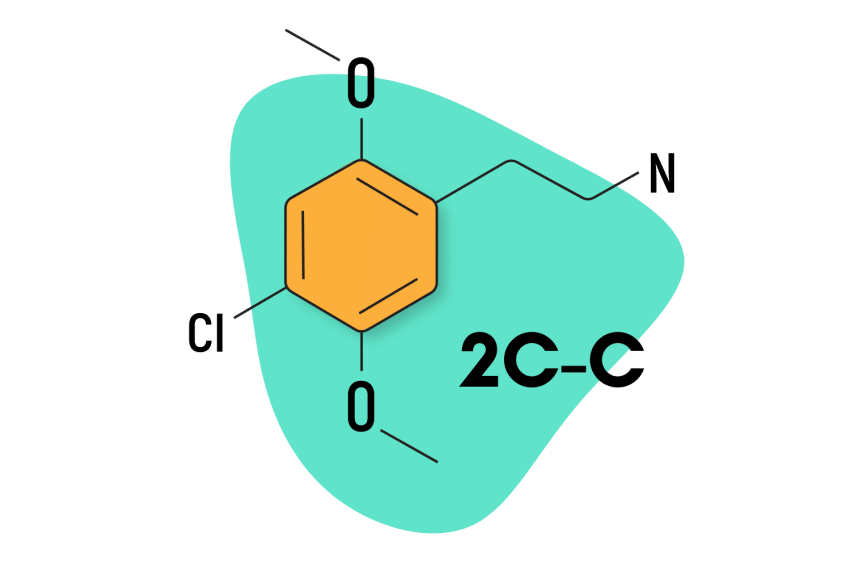
2C-C
2C-C has a relatively simple structure — featuring just one chlorine molecule in the R4 position.
The effects of this compound are milder and more coherent than most 2C drugs, but it can also be a bit sedative in higher doses. It’s more relaxing than 2C-B but maintains the euphoric and aphrodisiac qualities this group is known for.
2C-C is loved for its ability to maintain a coherent headspace while still providing euphoric, psychedelic, and empathogenic effects. What makes this compound unique is its notable relaxing (even sedative) effects on the body while maintaining feelings of mental alertness.

2C-C Specs:
| Chemical Name | 2,5-Dimethoxy-4-chlorophenethylamine |
| Status | Research Chemical 🧪 |
| Duration of Effects | 4–8 hours |
| Estimated Threshold Dose | 5 mg |
| Common Dose | 30–50 mg |
| PubChem ID | 29979100 |
| CAS# | 88441-14-9 |
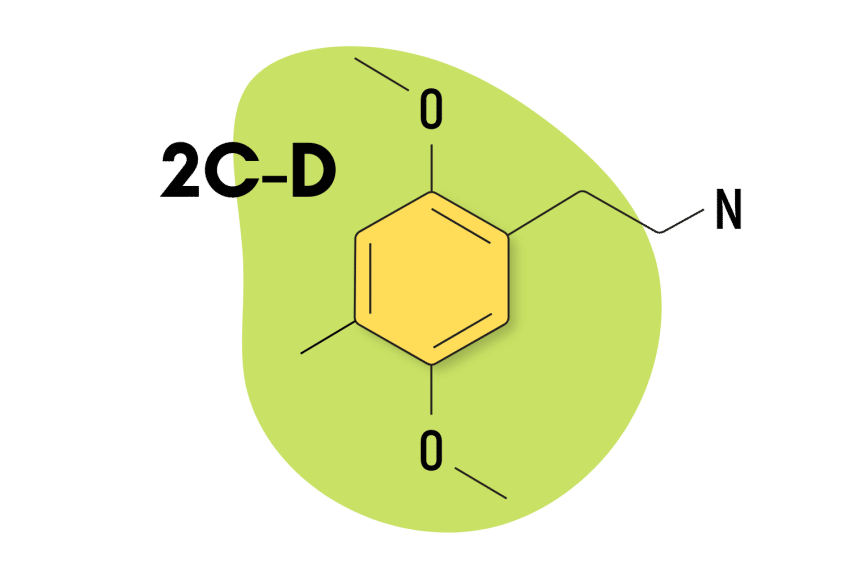
2C-D
2C-D (AKA 2C-M or LE-25) is known for its mild nature and short duration of effects. Shulgin referred to it as “pharmacological tofu” because he believed it could potentiate the psychedelic effects of other compounds but lacked any significant “psychedelic flavor” of its own.
Structurally, 2C-D features a methyl group at the R4 location. It’s an analog of another psychedelic compound called DOM (a substituted amphetamine psychedelic).

2C-D Specs:
| Chemical Name | 2,5-dimethoxy-4-methylphenethylamine |
| Status | Research Chemical 🧪 |
| Duration of Effects | 3–5 hours |
| Estimated Threshold Dose | 3 mg |
| Common Dose | 25–50 mg |
| PubChem ID | 135740 |
| CAS# | 24333-19-5 |
Compounds related to 2C-D include:
- 2C-DFM
- DOM
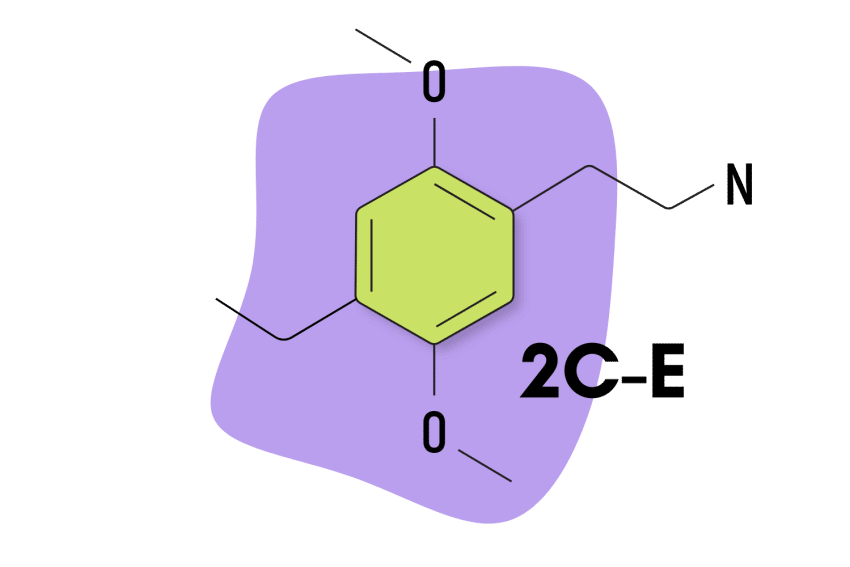
2C-E
2C-E is the third-most-popular member of the 2C psychedelic group, according to a 2019 study [2]. Its popularity is surprising due to the “challenging” nature associated with this compound.
The experience tends to follow an ebb and flow with increasing intensity, followed by periods of deep introspection. Some people find the effects to be too rapid and sudden, leading to “challenging” experiences.
Structurally, 2C-E contains an ethyl group on the R4 position.

2C-E Specs:
| Chemical Name | 2,5-Dimethoxy-4-ethylphenethylamine |
| Status | Research Chemical 🧪 |
| Duration of Effects | 8–12 hours |
| Estimated Threshold Dose | 2 mg |
| Common Dose | 10–15 mg |
| PubChem ID | 24729233 |
| CAS# | 71539-34-9 |
2C-F
2C-F is one of the least understood members of the 2C family. It was invented by Shulgin, but he didn’t leave any information regarding the effect profile.
Several users have reported the minimum psychoactive dose of this compound is around 250 mg or higher. This relatively high dose is likely the reason why this substance never became popular.
In general, fluorinated psychedelics are becoming increasingly popular. In the medical industry, fluorinated compounds can be found in as many as 15% of available medications [5]. The addition of fluorine has a high probability of altering the effects — usually increasing potency or duration.
In some cases, such as with 2C-F, this alteration actually makes it weaker instead. The minimum dose of 2C-F is reportedly around 250 mg — which is around 5 times less potent than most members of this group.

2C-F Specs:
| Chemical Name | 4-fluoro-2,5-dimethoxyphenethylamine |
| Status | Research Chemical 🧪 |
| Duration of Effects | Unknown |
| Estimated Threshold Dose | 150 mg |
| Common Dose | 250+ mg |
| PubChem ID | 44719499 |
| CAS# | 207740-15-6 |
2C-G
2C-G is one of the rarest members of the 2C psychedelic group. It was tested by Shulgin, but there hasn’t been much information published on this compound since then, and it’s notoriously difficult to find. The effects are reported to be stimulating like amphetamines and very long-lasting compared to other 2C substances. The trade-off is that 2C-G has much milder visual and tactile effects.
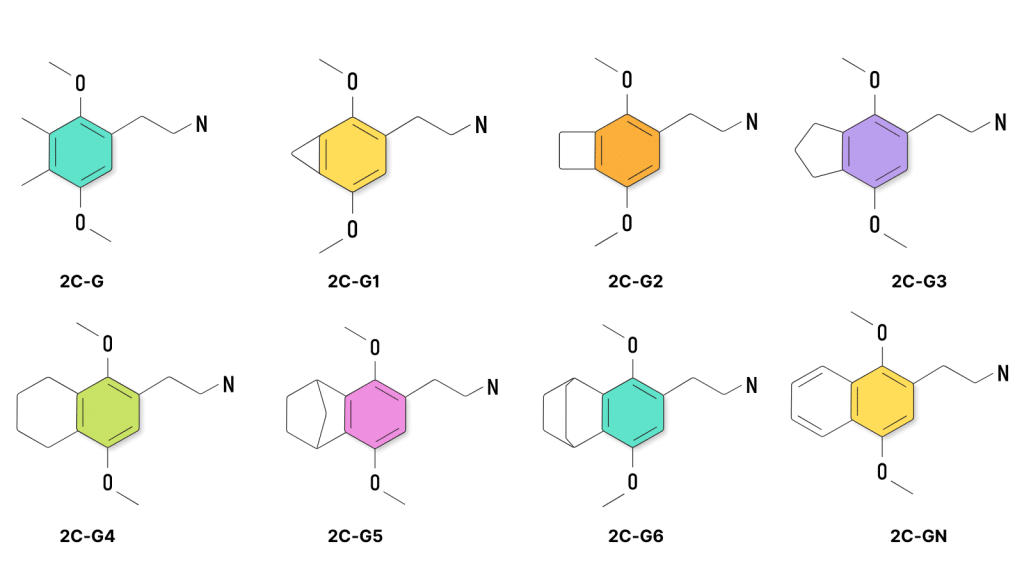
There are several different homologs of 2C-G — including 2C-G, 2C-G1, 2C-G2, 2C-G-3, 2C-G4, 2C-G5, 2C-G6, and 2C-GN.
G3, G5, and GN are available on the market, but G1, G2, G4, and G6 are substantially more difficult to make and are not easy to find.

2C-G Specs:
| Chemical Name | 3,4-dimethyl-2,5-dimethoxyphenethylamine |
| Status | Research Chemical 🧪 |
| Duration of Effects | 18–36 hours |
| Estimated Threshold Dose | 5 mg |
| Common Dose | 20–35 mg |
| PubChem ID | 22238091 |
| CAS# | 207740-18-9 |
2C-H
2C-H is the simplest member of the 2C group. It contains nothing but hydrogen in the R3 and R4 positions. This is essentially the most basic 2C molecule possible. It was first discovered in 1932 by Johannes S. Buck and is mainly used as the precursor for making other 2C substances.
This compound has very low, if any, psychoactive effects. It’s believed monoamine oxidase breaks this compound down before it’s able to exert any mind-altering effects.

2C-H Specs:
| Chemical Name | 2,5-dimethoxyphenethylamine |
| Status | Research Chemical 🧪 |
| Duration of Effects | Unknown |
| Estimated Threshold Dose | Unknown |
| Common Dose | Unknown |
| PubChem ID | 76632 |
| CAS# | 3600-86-0 |
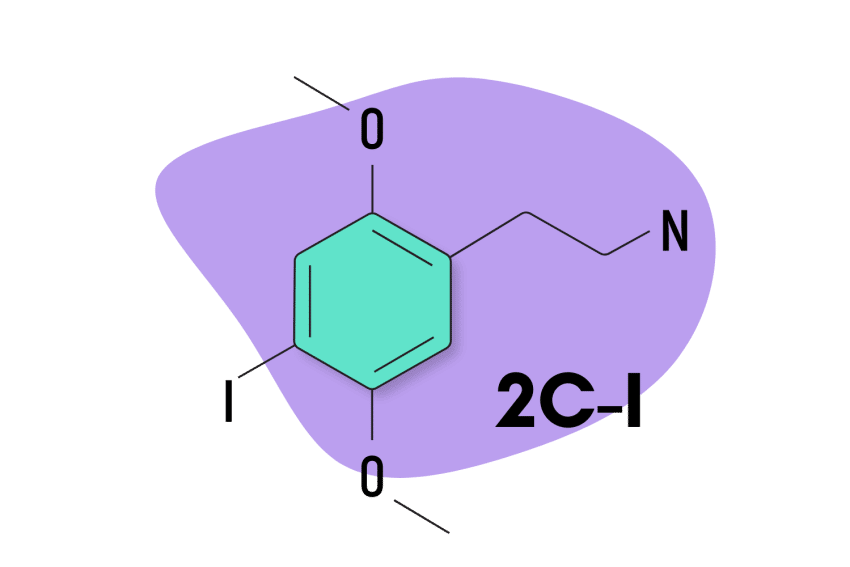
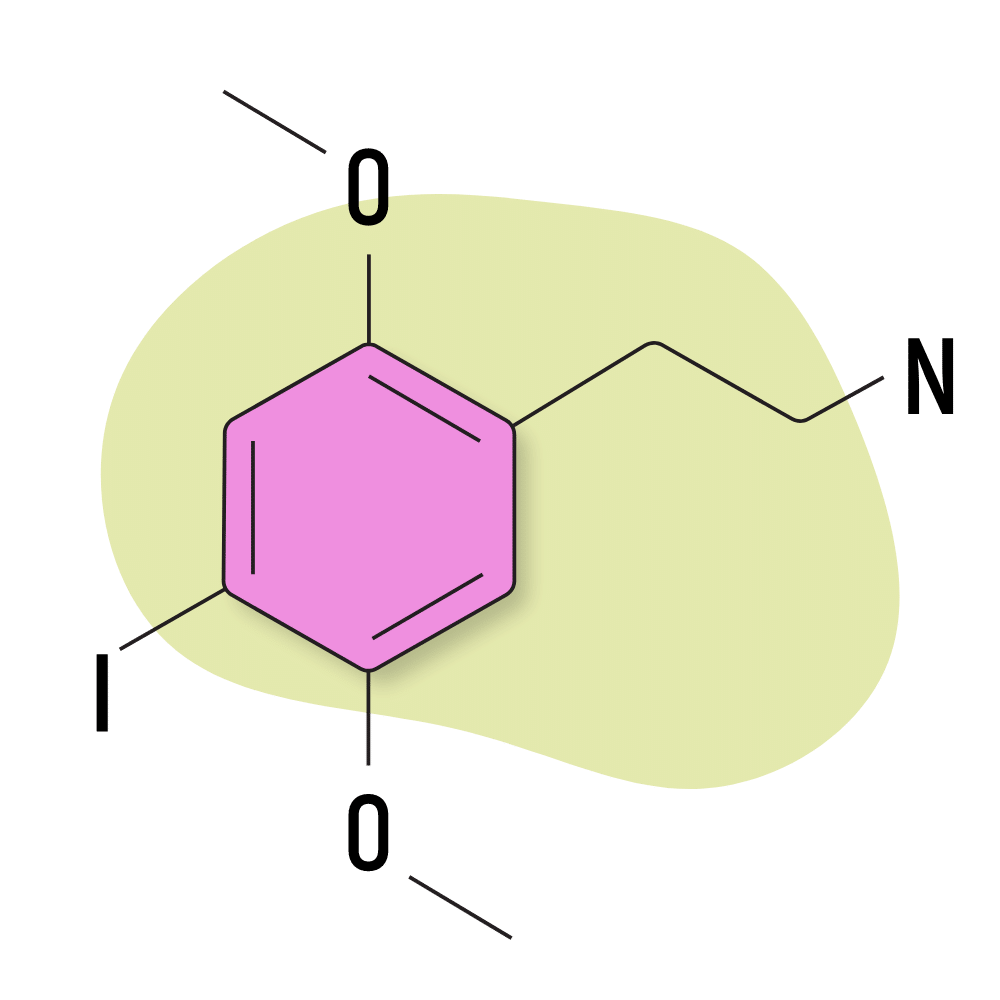
2C-I
2C-I is the second most popular and strongest member of the 2C psychedelic group. It’s preferred for its slow come-up, which makes it less likely to cause “challenging” experiences. People feel as though they’re eased into the experience rather than kicked straight into high gear, which can be very uncomfortable.
The catch is that the height of the experience tends to be stronger than other 2C substances. Some people still find the top of the 2C-I experience to be too strong for comfort.
The effects of 2C-I are moderately visual and entactogenic — facilitating a deeper sense of connection with others. This experience is common with the 2C substances.
Some people take sub-perceptual microdoses of 2C-I as a nootropic (less than 8 mg).

2C-I Specs:
| Chemical Name | 2,5-dimethoxy-4-iodophenethylamine |
| Status | Research Chemical 🧪 |
| Duration of Effects | 6–12 hours |
| Estimated Threshold Dose | 2 mg |
| Common Dose | 10–20 mg |
| PubChem ID | 10267191 |
| CAS# | 69587-11-7 |
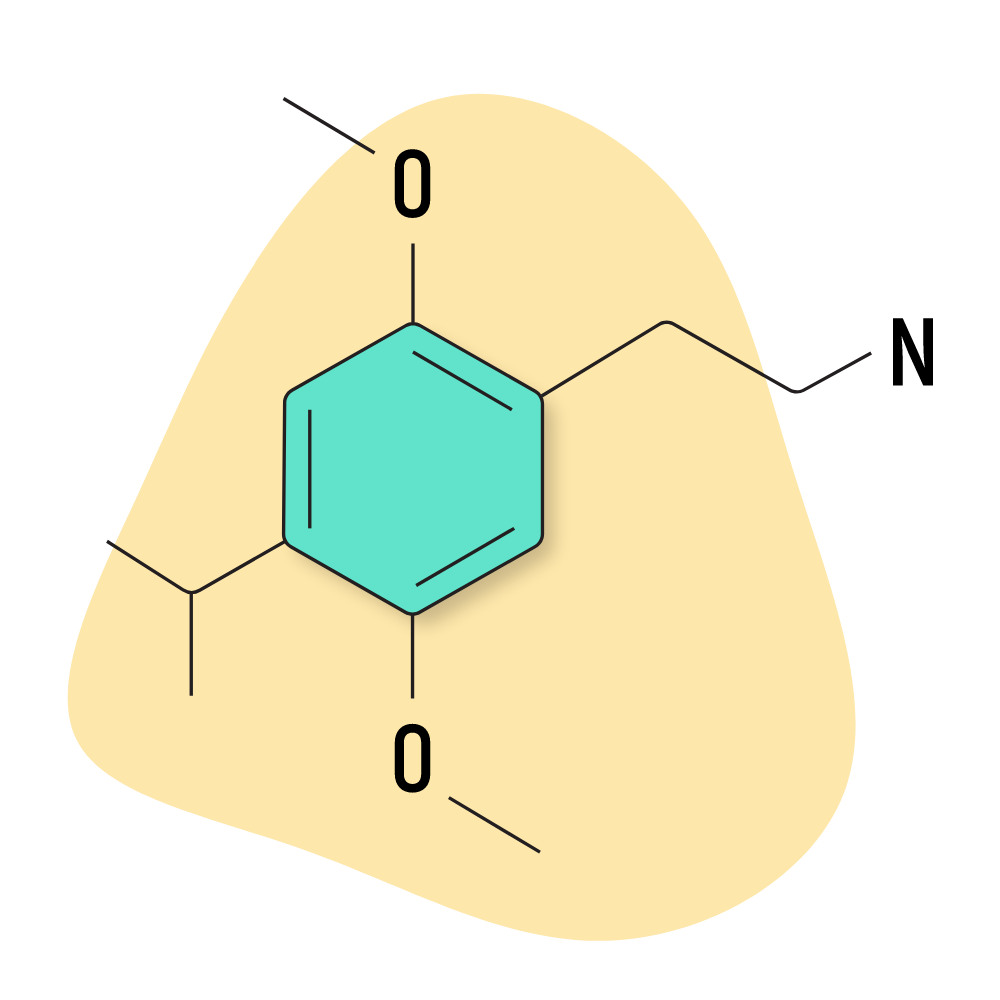
2C-iP
2C-iP (4-Isopropyl-2,5-dimethoxyphenethylamine) is the isopropyl isomer of 2C-P and reportedly offers similar, albeit “smoother” effects. The dose appears to be similar to 2C-P, which is one of the more potent 2CX compounds available. Tread cautiously with this one.
This compound was invented by Dmitri Ger who called the compound “Jelena” — however, 2C-iP is the more common name used today.

2C-iP Specs:
| Chemical Name | 4-Isopropyl-2,5-dimethoxyphenethylamine |
| Status | Research Chemical 🧪 |
| Duration of Effects | Unknown |
| Estimated Threshold Dose | Unknown |
| Common Dose | Unknown |
| PubChem ID | 57474311 |
| CAS# | 1498978-47-4 |
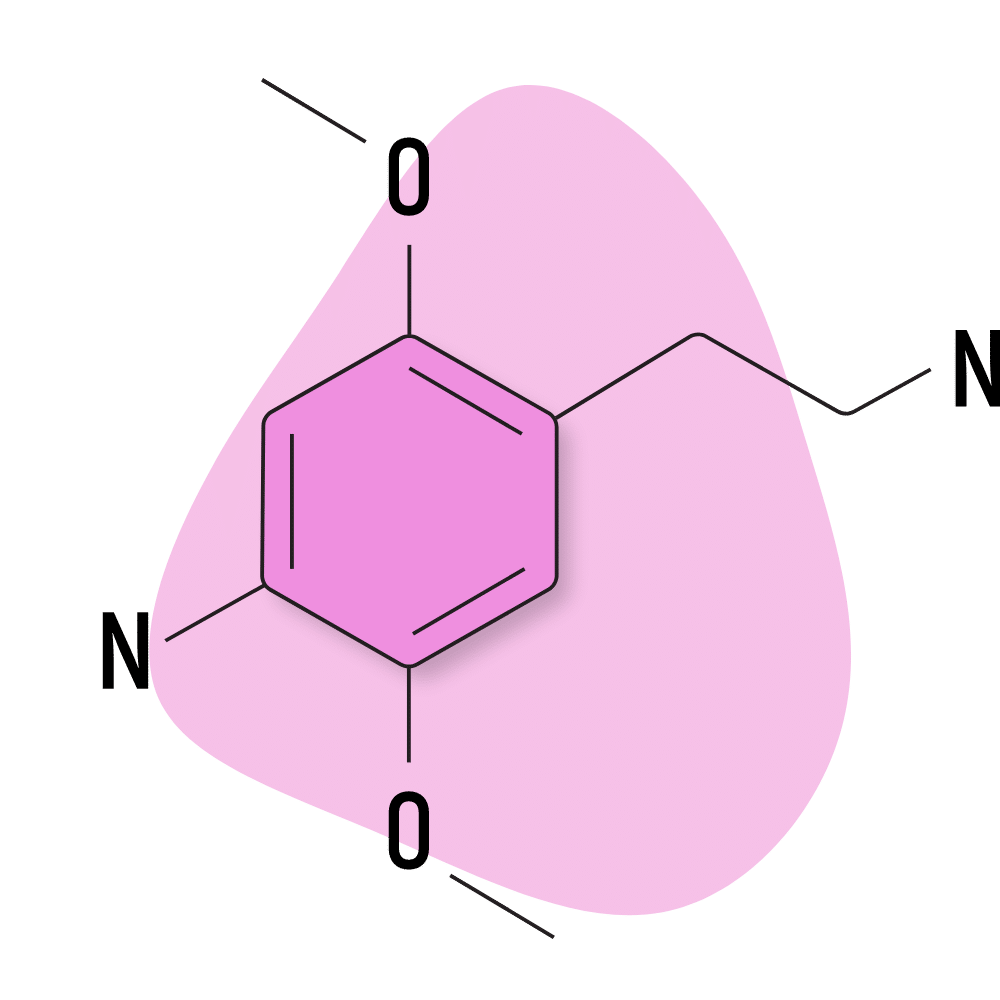
2C-N
2C-N is not well studied, and there are very few trip reports available for this compound online. Most of the reports suggest it to be similar but inferior to the effects of MDMA.
2C-N is characterized by a nitro group on the R4 position.

2C-N Specs:
| Chemical Name | 2,5-dimethoxy-4-nitrophenethylamine |
| Status | Research Chemical 🧪 |
| Duration of Effects | 4–6 Hours |
| Estimated Threshold Dose | 25 mg |
| Common Dose | 100–150 mg |
| PubChem ID | 10036637 |
| CAS# | 261789-00-8 |
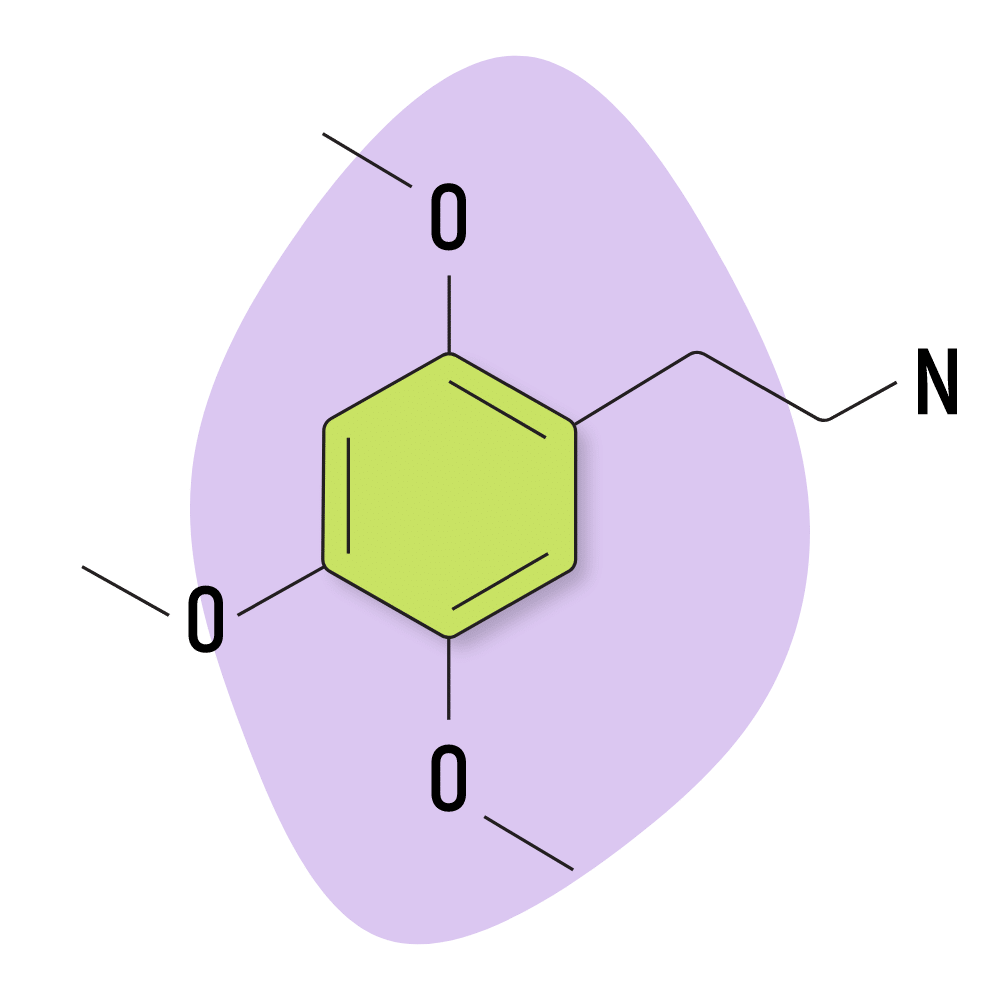
2C-O
From the limited information available for this compound (basically just Shulgin’s notes), this compound has very low activity. In doses over 300 mg, the effects were reported as somewhat similar to mescaline, but with more nausea and less euphoria.

2C-O Specs:
| Chemical Name | 2,4,5-trimethoxyphenethylamine |
| Status | Research Chemical 🧪 |
| Duration of Effects | Unknown |
| Estimated Threshold Dose | Unknown |
| Common Dose | Unknown |
| PubChem ID | 151954 |
| CAS# | 15394-83-9 |
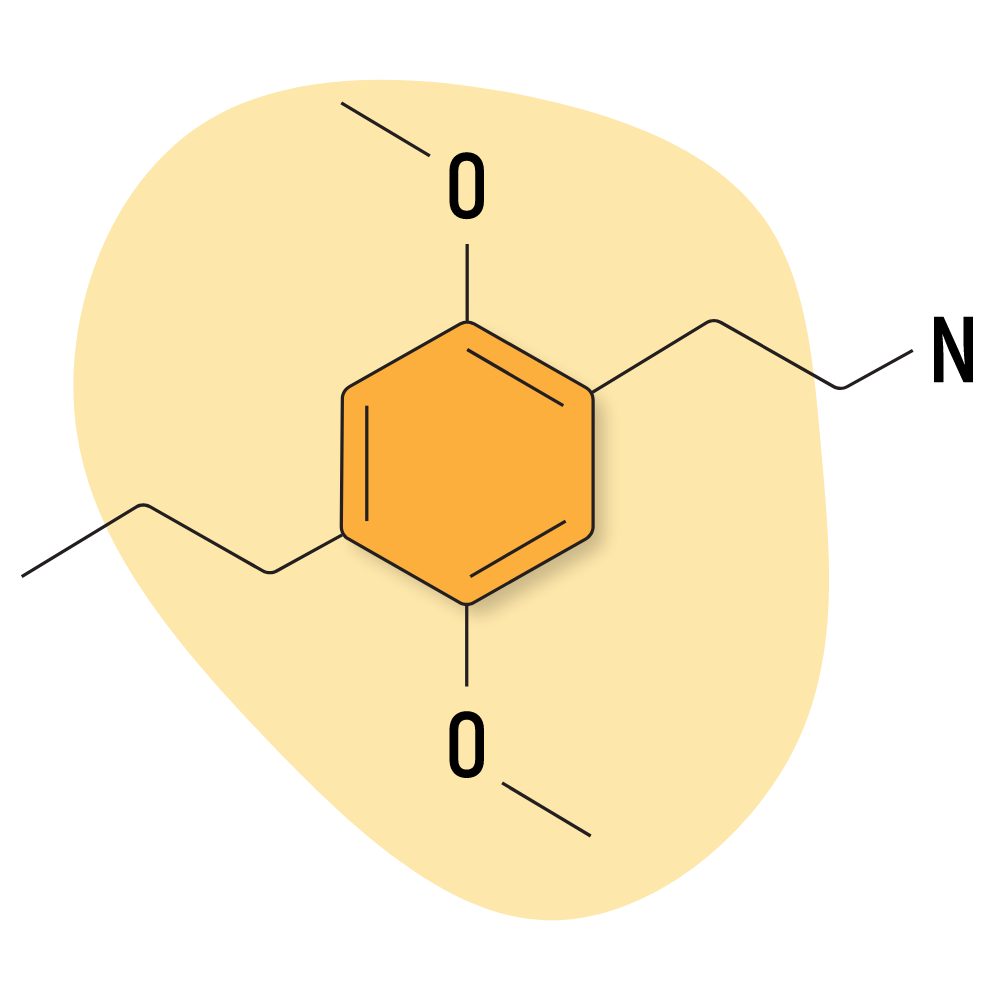
2C-P
2C-P is a lesser-known member of the 2C family that’s become more popular in recent years. It’s revered for its very high potency and long duration of effects. The peak is reported to last as long as 10 hours, for a total duration of around 16–20 hours.
Like most other 2C compounds, the effects are euphoric and stimulating in lower doses and intensely psychedelic in higher doses.
The functional group on 2C-P is a propyl group on the R4 position.

2C-P Specs:
| Chemical Name | 2,5-dimethoxy-4-propylphenethylamine |
| Status | Research Chemical 🧪 |
| Duration of Effects | 16–20 hours |
| Estimated Threshold Dose | Unknown |
| Common Dose | Unknown |
| PubChem ID | 44350080 |
| CAS# | 207740-22-5 |
Similar Compounds:
- 2C-iP (structural analog)
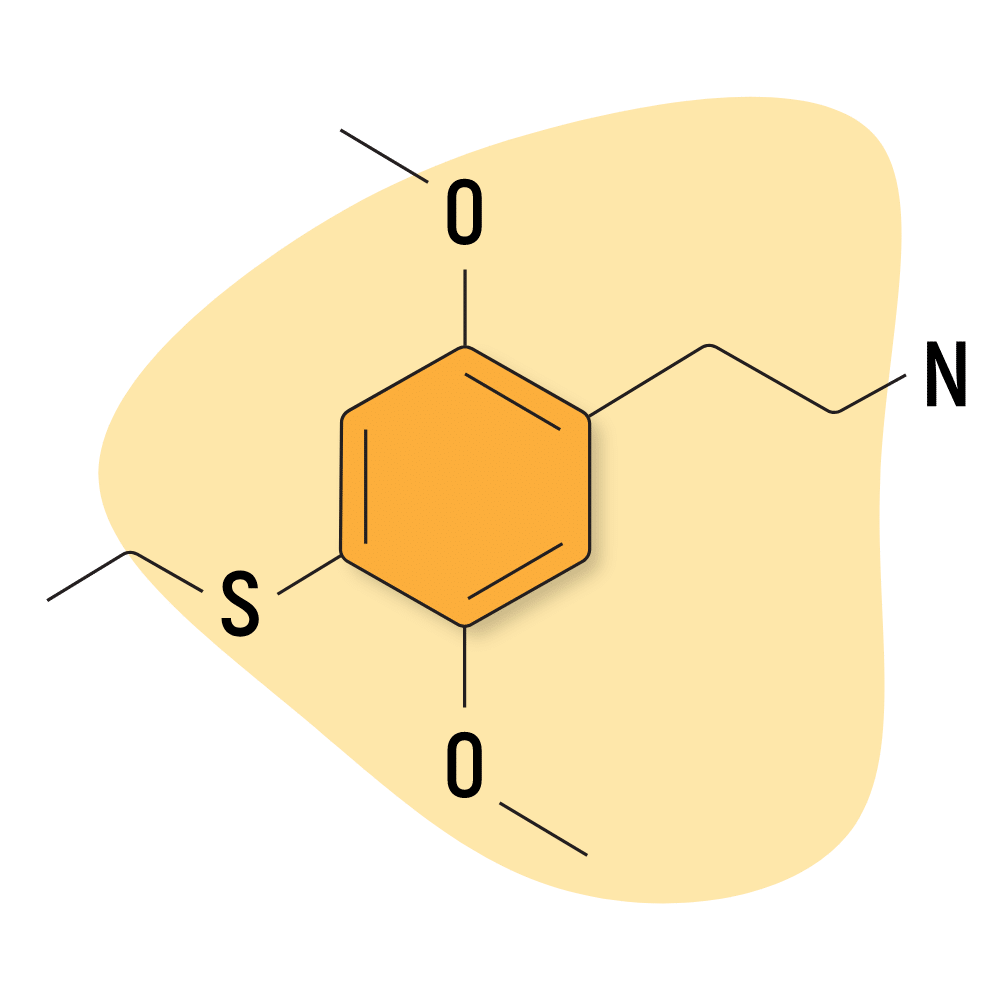
2C-T-2
The 2C-T-X compounds are a subgroup of the 2C family. These compounds feature various sulfide groups at the R4 position. Only about half of these compounds are psychoactive; the other half appear to be inactive.
Inactive members of the 2C-T group include 2C-T-3, 5, 6, 10, 11, 12, 14, 16, 18, 19, 20, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, and 32.
Psychoactive members of the 2C-T family include 2C-T-2, 4, 7, 8, 9, 13, 15, 17, and 21.
The most popular members of the group are 2C-T-2 and 2C-T7 — both of which are included in Alexander Shulgin’s “magical half-dozen.”
2C-T-2 is known for its potent euphoric qualities, similar to MDMA.

2C-T-2 Specs:
| Chemical Name | 2,5-Dimethoxy-4-ethylthiophenethylamine |
| Status | Research Chemical 🧪 |
| Duration of Effects | 6–8 hours |
| Estimated Threshold Dose | 3 mg |
| Common Dose | 10–20 mg |
| PubChem ID | 12074193 |
| CAS# | 207740-24-7 |
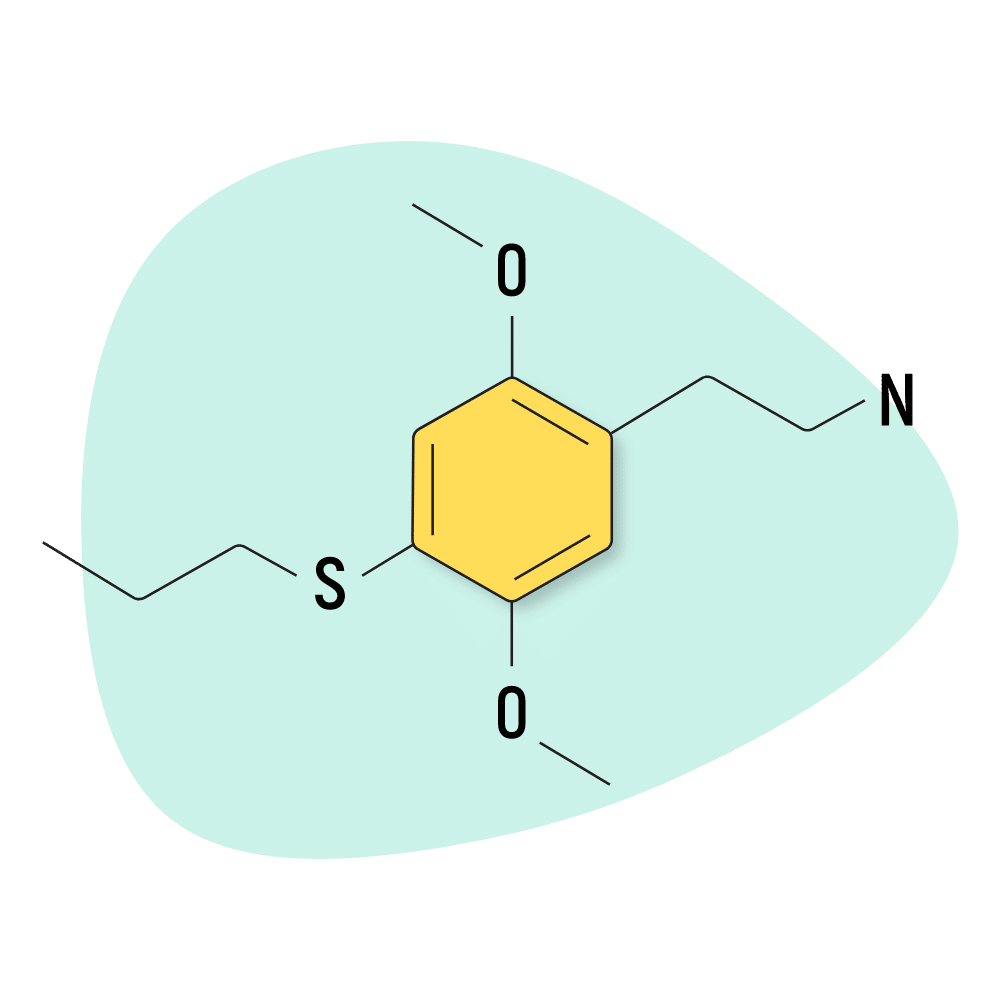
2C-T-7
2C-T-7 is the other “popular” 2C-T-X compound — best known for its long-lasting effects (up to 15 hours) and consistent euphoria. This compound is strong, but it’s less likely to result in “challenging” or “uncomfortable” experiences than many other 2C compounds.
This substance is listed as one of Shulgin’s favorite inventions.

2C-T-7 Specs:
| Chemical Name | 2,5-Dimethoxy-4-propylthiophenethylamine |
| Status | Research Chemical 🧪 |
| Duration of Effects | 8–15 hours |
| Estimated Threshold Dose | 3 mg |
| Common Dose | 15–25 mg |
| PubChem ID | 24728635 |
| CAS# | 207740-26-9 |
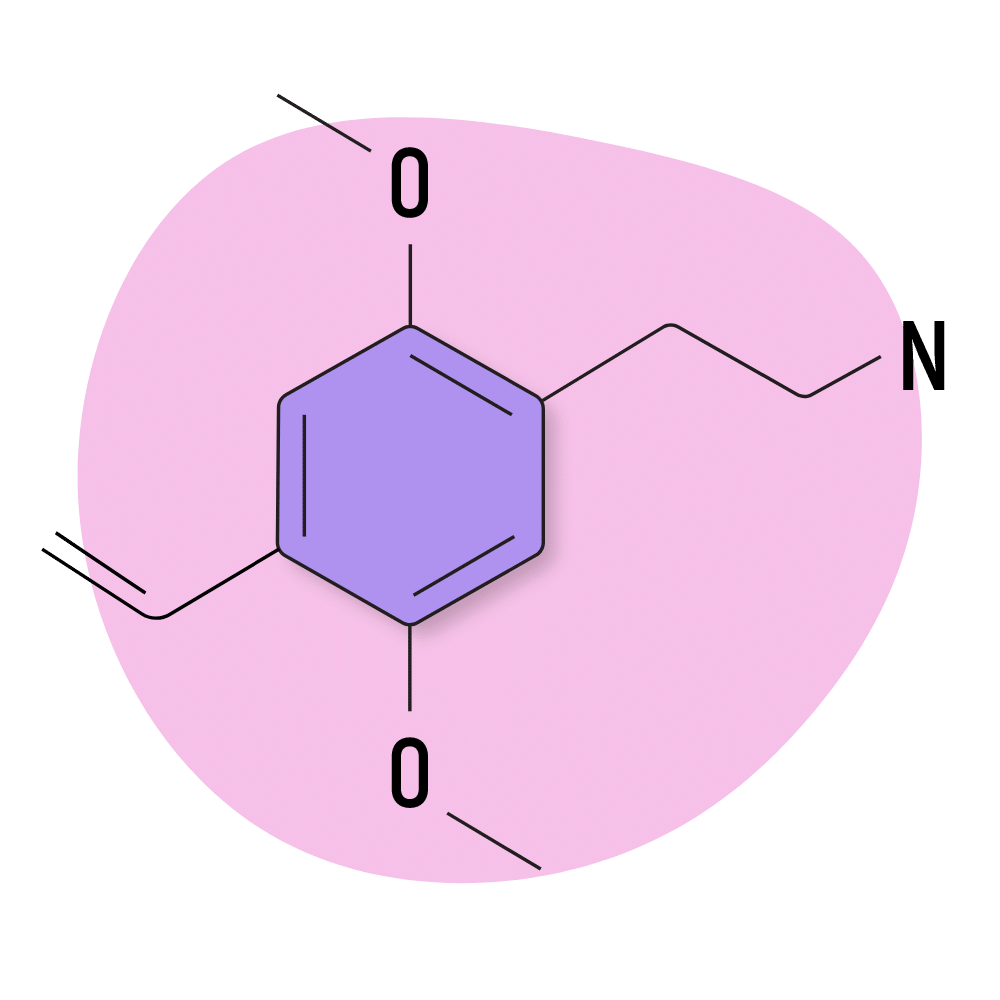
2C-V
2C-V (2-(4-ethenyl-2,5-dimethoxyphenyl)ethanamine) uses vinyl for the functional group. It was invented by Daniel Trachsel in 2006 [6] but ultimately never made it to the designer drug market. It’s both hard to make and unstable, which provides poor incentive for manufacturers.
The threshold dose of this substance is reported by Tranchsel to be around 25 mg and last approximately 5 hours.

2C-V Specs:
| Chemical Name | 2-(4-ethenyl-2,5-dimethoxyphenyl)ethanamine |
| Status | Research Chemical 🧪 |
| Duration of Effects | 5 hours |
| Estimated Threshold Dose | 5 mg |
| Common Dose | 25–50 mg |
| PubChem ID | 57474284 |
| CAS# | N/A |
Other Members of the 2C Group
There are dozens of other 2C compounds that have already been “discovered” but lack enough testing to provide much context about how they feel or what the threshold dose looks like. There are likely countless others yet to be created.
Here’s a list of other 2C substances that don’t have enough information available to draw any useful conclusions in terms of their effects but are worth knowing about nonetheless:
- 2C-AL
- 2C-Bn
- 2C-Bu
- 2C-C3
- 2C-CN
- 2C-CP
- 2C-DFM
- 2C-MOM
- 2C-NH2
- 2C-EF
- 2C-Ph
- 2C-PIP
- 2C-PYR
- 2C-Se
- 2C-TFE
- 2C-TFM
- 2C-YN
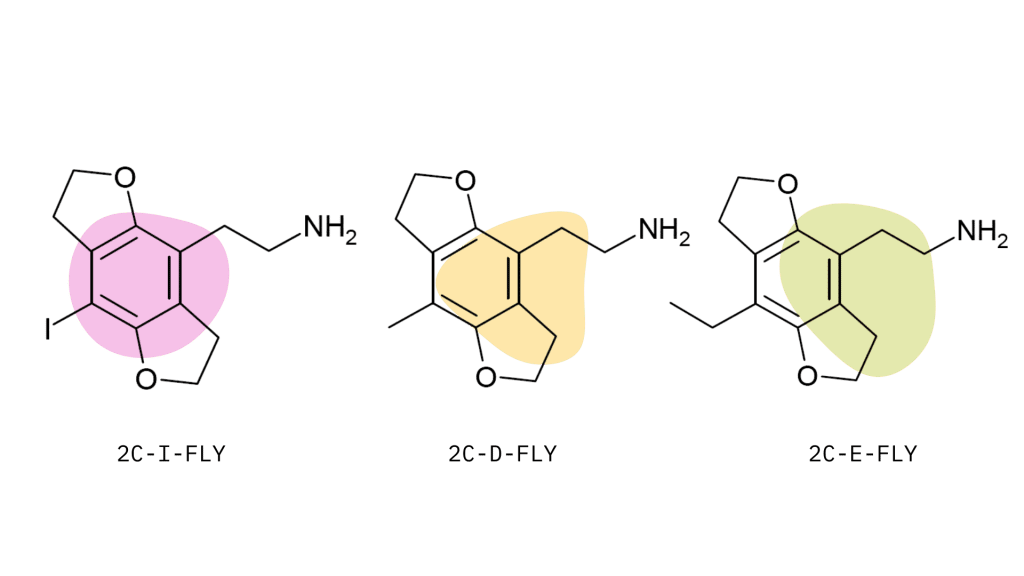
Substituted Benzofuran Compounds (2C-X-FLY)
There are a variety of 2C substances that have been dihydrodifuranated. Many of these compounds are considered to be more potent than the original 2C substance they were derived from.
Dihydrodifuranated analogs often contain the word FLY in the name, such as 2C-B-FLY, 2C-C-FLY, etc.
There are also benzofuran substitutions of DOX compounds, amphetamines, mescaline, and much more.
List of popular dihydrodifuranated 2C psychedelics:
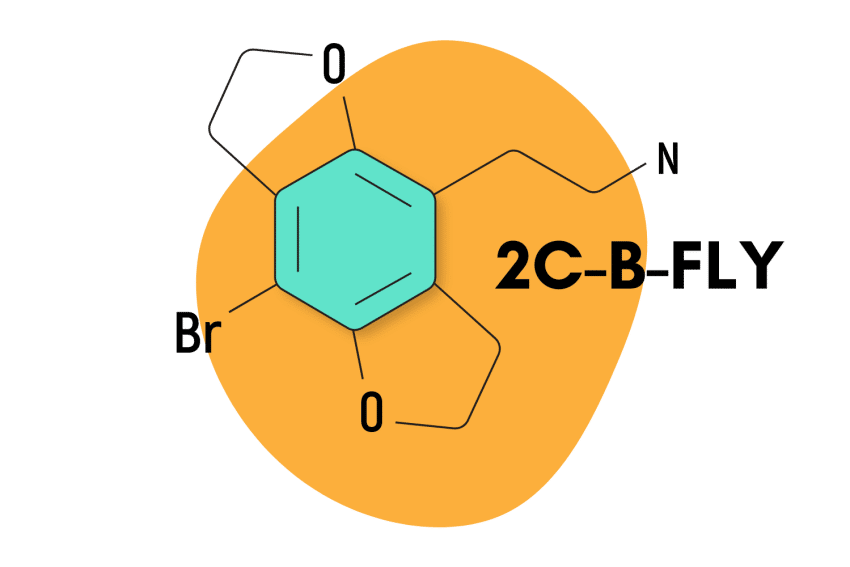
2C-B-FLY
2C-B-FLY is considered milder than 2C-B in that users claim to retain a clearer mental headspace. The peak effects are less intense with 2C-B-FLY, but the body load tends to be a bit stronger, and the duration is about 20% longer.
This psychedelic is formidable and can still catch users off-guard if they aren’t careful.

2C-B-FLY Specs:
| Chemical Name | Chemical Name: 8-bromo-2,3,6,7-benzo-dihydro-difuran-ethylamine |
| Status | Research Chemical 🧪 |
| Duration of Effects | 7–12 Hours |
| Estimated Threshold Dose | 2 mg |
| Common Dose | 10–18 mg |
| PubChem ID | 10265873 |
| CAS# | 733720-95-1 |
2C-B-DRAGONFLY
2C-B-DRAGONFLY is roughly 2 or 3 times stronger than 2C-B and nearly 4 or 5 times stronger than 2C-B-FLY. Its fully aromatic benzodifuran rings are thought to enhance the binding capacity of the molecule compared to the basic 2C-B molecule.
An even stronger relative, Bromo-DRAGONFLY, also exists, but there’s very little information about this substance in the online literature.

2C-B-DRAGONFLY Specs:
| Chemical Name | 1-(8-Bromo-dibenzo[1,2-b; 4,5-b’]difuran-4-yl-2-aminoethane |
| Status | Research Chemical 🧪 |
| Duration of Effects | Unknown |
| Estimated Threshold Dose | Unknown |
| Common Dose | Unknown |
| PubChem ID | 91753712 |
| CAS# | 260809-98-1 |
2C-B-BUTTERFLY
Sometimes called 2C-B-MOTH, this compound is comparable in terms of potency to 2C-B-FLY. Some early studies have shown that this molecule has a higher affinity for the 5HT2C receptors compared to the 5HT2A receptors. Both serotonin receptors are involved in producing psychedelic, mood-altering, and energizing effects.

2C-B-BUTTERFLY Specs:
| Chemical Name | 2-(10-Bromo-2,3,4,7,8,9-hexahydro-pyrano[2,3-g]chromen-5-yl)-ethylamine |
| Status | Research Chemical 🧪 |
| Duration of Effects | Unknown |
| Estimated Threshold Dose | Unknown |
| Common Dose | Unknown |
| PubChem ID | 10244981 |
| CAS# | 502659-24-7 |
2C-C-FLY
Very little information is available about 2C-C-FLY. It’s hard to make and doesn’t produce nearly as potent psychedelia as most of the other 2C substances.
Some reports suggest 2C-C-FLY is even more sedative and lucid than 2C-C, which some people may find enticing.
The safety of this compound, as with many other 2C compounds, is unknown.

2C-C-FLY Specs:
| Chemical Name | 2-(8-Methyl-2,3,6,7-tetrahydro-1,5-dioxa-s-indacen-4-yl)ethylamine |
| Status | Research Chemical 🧪 |
| Duration of Effects | Unknown |
| Estimated Threshold Dose | Unknown |
| Common Dose | Unknown |
| PubChem ID | 115010668 |
| CAS# | 1354633-83-2 |
Other 2C-X-FLY (Benzofuran) Compounds
Virtually any 2C compound can be dihydrofurinated, but most are either not worth it or simply haven’t been done publicly.
Here are some of the other FLY compounds that have been created so far:
- 2C-D-FLY
- 2C-E-FLY
- 2C-EF-FLY
- 2C-I-FLY
- 2C-T-7-FLY
- Mescaline-FLY (sometimes called “bat”)
25X-NBX (NBOMe) Compounds
NBOMe compounds (called N-bombs) are a group of highly psychoactive, synthetic, substituted phenethylamines compounds derived from the 2C family. They were first discovered in the early 2000s by Ralf Heim at the Free University of Berlin [4].
The NBOMe compounds mainly consist of N-benzylphenethylamines, but sometimes feature other functional groups at the position of the N-benzyl group. Some compounds substitute thiophene, pyridine, furan, tetrahydrofuran, benzodioxole, or naphthalene instead.
Most 2C compounds have an NBOMe version, including NBOMe-mescaline, 25B-NBOMe, 25C-NBOMe, 25H-NBOMe, 25I-NBOME, and so on (there are dozens of them).
By far, the strongest and most common member of the group is 25I-NBOMe, which is a substituted version of 2C-I (also the strongest member of the 2C family). 25I-NBOMe is one of the few compounds that deliver psychoactive effects in sub-milligram doses. This allows it to be dosed on blotter paper and sold under the guise of LSD. Most other drugs wouldn’t be potent enough to pass as LSD in such low doses.
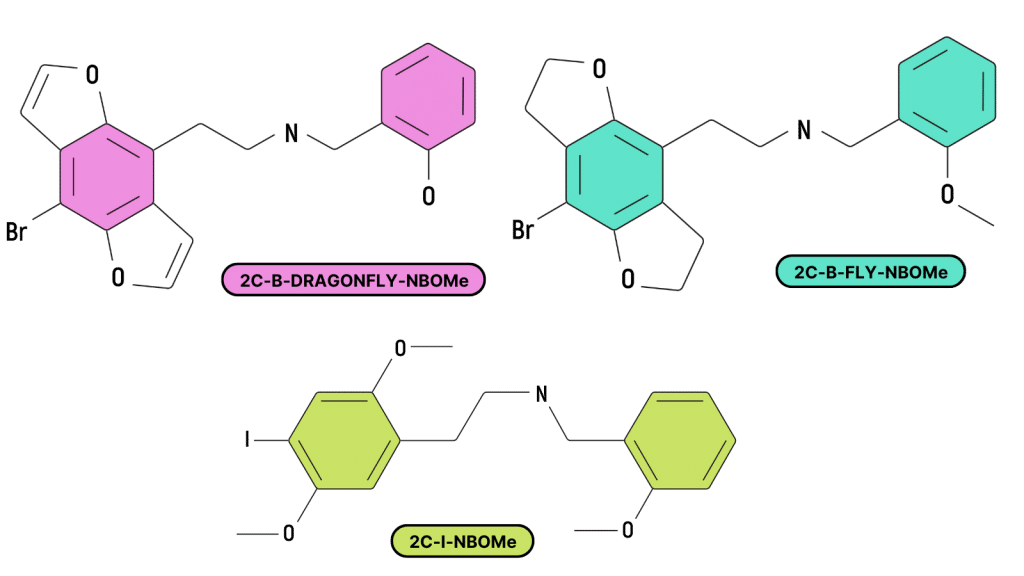
Other common 25X-NBX compounds include 25B-NBOMe and 25C-NBOMe. These three substances were reportedly found in approximately 0.03% of the total hallucinogen samples seized globally between 2011 and 2017.
Unfortunately, NBOMe compounds are notoriously dangerous. They have a delayed onset of effects and a narrow dosage window. Numerous people have died from taking NBOMe substances over the years — often while using doses believed to be safe. Everyone reacts differently to these compounds, and there are cases of people dying after taking just one tab.
Are 2C Substances Safe? What Are The Risks?
Most 2C compounds appear to be relatively safe. Shulgin himself tested over 60 of these substances without any serious reactions reported.
With that said, some 2C compounds are very potent and can produce nasty side effects if too much is taken. Many members of this family produce uncomfortable body load if too much is taken at once. This can involve feelings of nausea, cramping, muscle tension, and abdominal pain lasting anywhere from a few minutes to a few hours.
It’s also relatively common to experience a bit of a hangover the following day, especially with higher doses.
Some members, such as 2C-I-NBOMe, have been associated with fatal overdose. It’s wise to avoid NBOMe compounds of any kind because of this effect.
Are 2C Compounds Legal?
No, 2C-X psychedelics are illegal in most countries. They were officially banned in the US in 1995 (starting with 2C-B) and added to the UN Convention of Psychotropic Substances six years later in 2001. As a result, all UN member countries have imposed similar bans on the 2C family of substances.
In Canada, 2C compounds are classified as Schedule III, which permits their use for research purposes.
There’s no current push toward legalizing this family of substances anytime soon.
How Do 2C Compounds Work?
2C substances are modeled after mescaline and are thought to work through similar mechanisms.
Mescaline works by activating the 5HT2A receptors in the brain. This causes a cascade of effects in the brain, leading to what we perceive as the psychedelic experience. Many psychedelics work through the same mechanism, including LSD, psilocybin, and DMT.
Compared to tryptamine-based psychedelics (LSD, psilocybin, DMT), 2Cs and other phenethylamines have a weaker action on the 5HT2A receptors. This suggests there are other mechanisms involved with the psychoactive effects. Some studies have found that 2C-B, the most studied member of the family, also affects the adrenergic receptors [3]. This action is likely the cause of the stimulating effects common in this group.
What’s The Strongest Member of the 2C-X Psychedelic Group?
The most potent member of the 2C family of drugs is 2C-I, followed by 2C-B and 2C-E.
The weakest psychoactive members of the group are 2C-D and 2C-O. Many others are non-psychoactive entirely, including about half the 2C-T group and a few of the 2C-G compounds.
The longest-lasting 2C compound is 2C-P.
What Were Shulgin’s Magical Half-Dozen?
Dr. Alexander Shulgin is the genius behind most of the 2C family of compounds, along with many other phenethylamine and tryptamine psychedelics. He would create and then test each of these substances with his wife, Anne Shulgin, and a group of friends to characterize their effects.
The Shulgin’s published their findings, along with the recipes to create each compound, in the book PIHKAL (Phenethylamines I Have Known And Loved). In it, they list their favorite compounds, which were aptly named the “magical half-dozen.” Most of these compounds are 2Cs, with the exception of mescaline (the starting point for all 2Cs, and DOM, which is an amphetamine).
Shulgin’s magical half-dozen includes:
What Are 3C Psychedelics?
While the name suggests 3C and 2C compounds are related, 3C compounds are technically classified as amphetamines.
Both 2C substances and amphetamines are members of the larger phenethylamine group but feature differences that alter the way these molecules are able to bind to receptors in the brain. Amphetamines tend to have a higher affinity for the dopamine receptors, while 2C prefers the serotonin receptors.
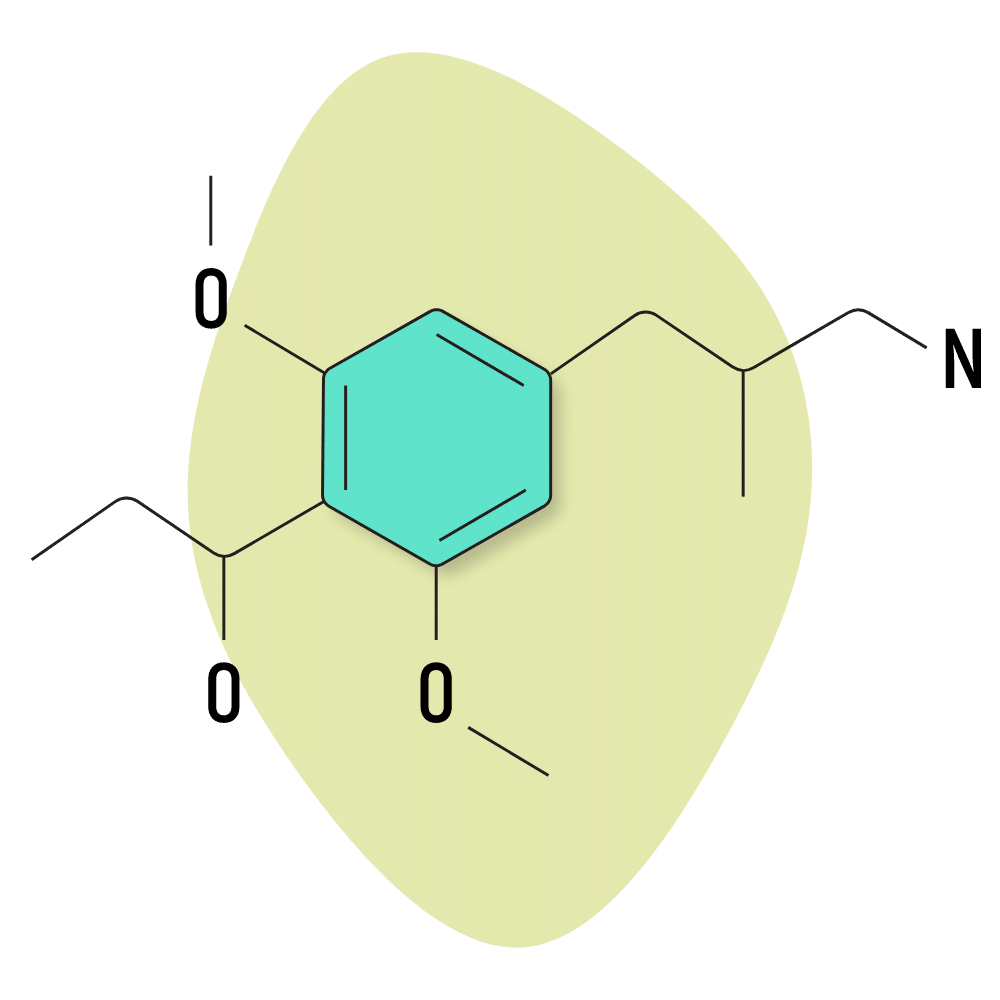
The most common 3C psychedelic is 3C-E, which is a 3-carbon analog of escaline.
Much like 2C compounds, the most prominent 3C substances were discovered by Alexander Shulgin.
Summary: What is 2C-X?
2C compounds (AKA 2C-X or 2CX) are a large and diverse group of phenethylamine-based psychedelics. They share the core structure of the natural psychedelic mescaline. As such, the effects of 2C substances are very similar to mescaline — producing strong visual, auditory, and tactile sensations.
Most 2C compounds provide a blend of hallucinogenic and empathogenic or euphoriant qualities. Some are more visual, some more tactile, and some more emotional or introspective in nature.
The most common 2C substances include 2C-B (the creator, Alexander Shulgin’s personal favorite), 2C-I (the strongest member of the class), and 2C-E.
There’s an ever-expanding list of substituted versions of 2C compounds, including benzofurinated or NBOMe compounds available too.
Overall, this interesting and valuable family of psychedelics has a lot to offer. There’s evidence to suggest some 2C compounds could be used in psychedelic-assisted psychotherapy, and some claim microdosing 2C substances are useful as a source of nootropic compounds.
Remember that most of the substances on this list are considered research chemicals — which means they lack crucial scientific research to prove them safe for human consumption.
If you have any comments or feedback that would improve the information in this resource, please email feedback@tripsitter.com.
References
- Stoller, A., Dolder, P. C., Bodmer, M., Hammann, F., Rentsch, K. M., Exadaktylos, A. K., … & Liakoni, E. (2017). Mistaking 2C-P for 2C-B: what a difference a letter makes. Journal of analytical toxicology, 41(1), 77-79.
- Sexton, J. D., Crawford, M. S., Sweat, N. W., Varley, A., Green, E. E., & Hendricks, P. S. (2019). Prevalence and epidemiological associates of novel psychedelic use in the United States adult population. Journal of Psychopharmacology, 33(9), 1058-1067.
- Villalobos, C. A., Bull, P., Sáez, P., Cassels, B. K., & Huidobro-Toro, J. P. (2004). 4-Bromo-2, 5-dimethoxyphenethylamine (2C-B), and structurally related phenylethylamines are potent 5-HT2A receptor antagonists in Xenopus laevis oocytes. British Journal of Pharmacology, 141(7), 1167-1174.
- Zawilska, J. B., Kacela, M., & Adamowicz, P. (2020). NBOMes–highly potent and toxic alternatives of LSD. Frontiers in Neuroscience, 78.
- Trachsel, D. (2012). Fluorine in psychedelic phenethylamines. Drug testing and analysis, 4(7-8), 577-590.
- Trachsel, D., Lehmann, D., & Enzensperger, C. (2013). Phenethylamine: von der Struktur zur Funktion. Nachtschatten-Verlag.