Lysergamide Psychedelics
LSD isn’t unique. There are dozens of related compounds that provide similar effects.
LSD is the most famous member of the lysergamides, but there are dozens more, many of which produce similar psychedelic effects.
Some lysergamides (including LSD) are useful for treating headaches, inducing labor, or treating other medical disorders in lower doses.
Here, we’ll take a deep dive into the world of lysergamide psychedelics. We’ll compare their effects, analyze what research is available, and provide details on what makes each of these intriguing chemicals unique.
What Are Lysergamide Psychedelics?
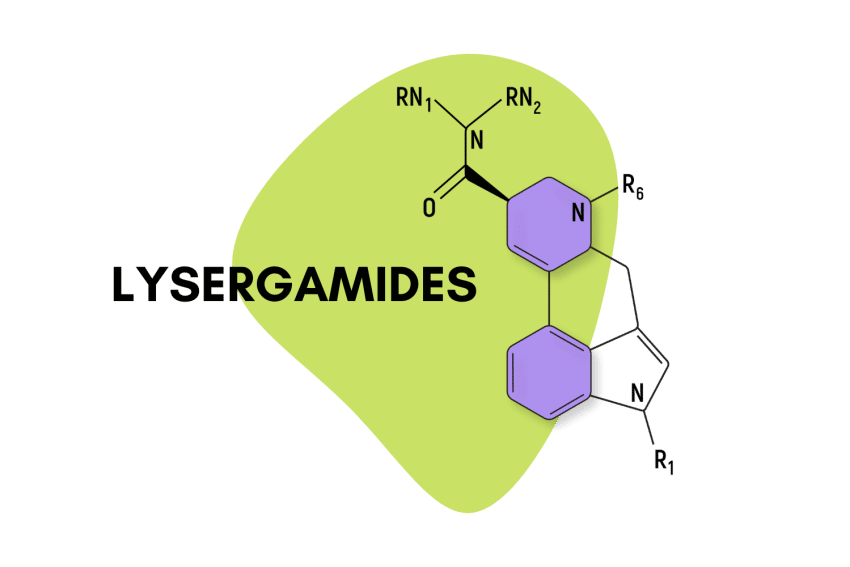
“Lysergamide” specifically refers to compounds that feature a lysergic acid base with an amide group attached.
Lysergamides mimic the effects of serotonin by binding to specific receptors in the brain to produce the characteristic psychedelic effects. The primary target is the 5HT2A serotonin receptor, but lysergamides will also interact with dopamine and norepinephrine to produce changes in mood, mental stimulation, and executive functioning.
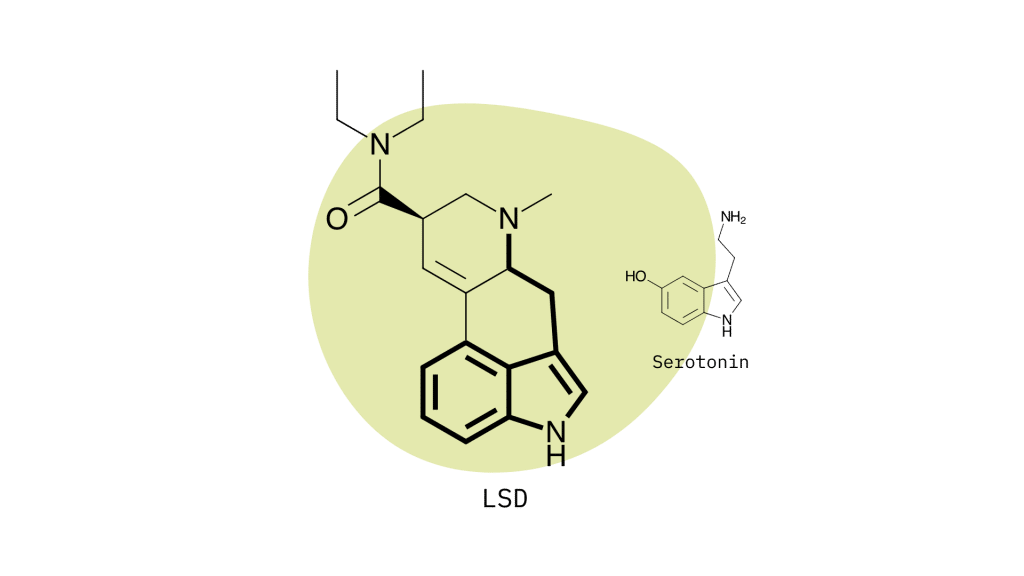
Lysergamide Chemical Structure
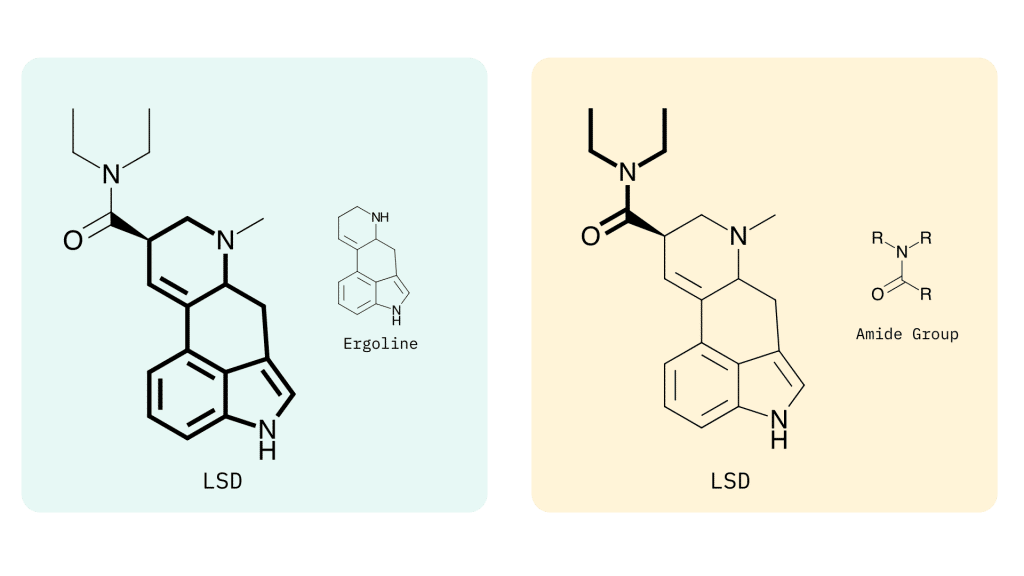
Lysergamides are polycyclic amides that have both phenethylamine and tryptamine groups integrated within the structure.
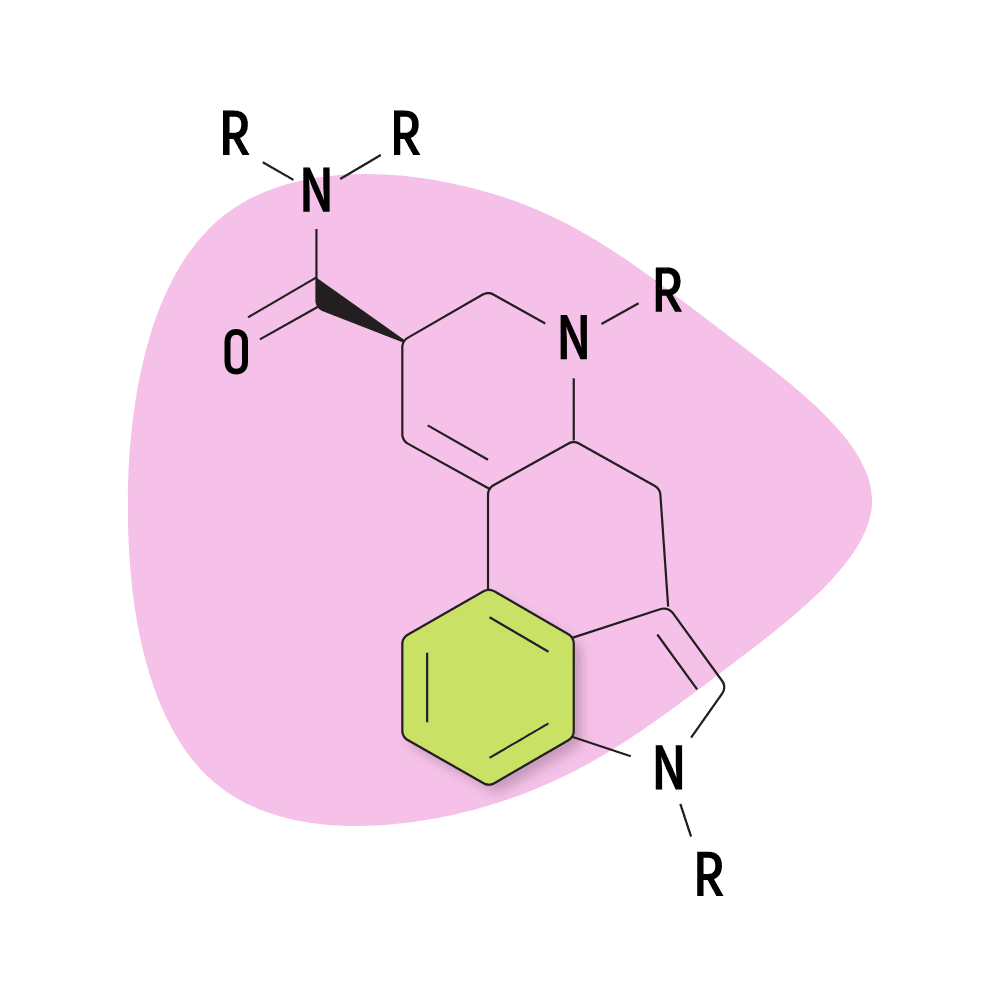
The base structure consists of ergoline and an amide group.
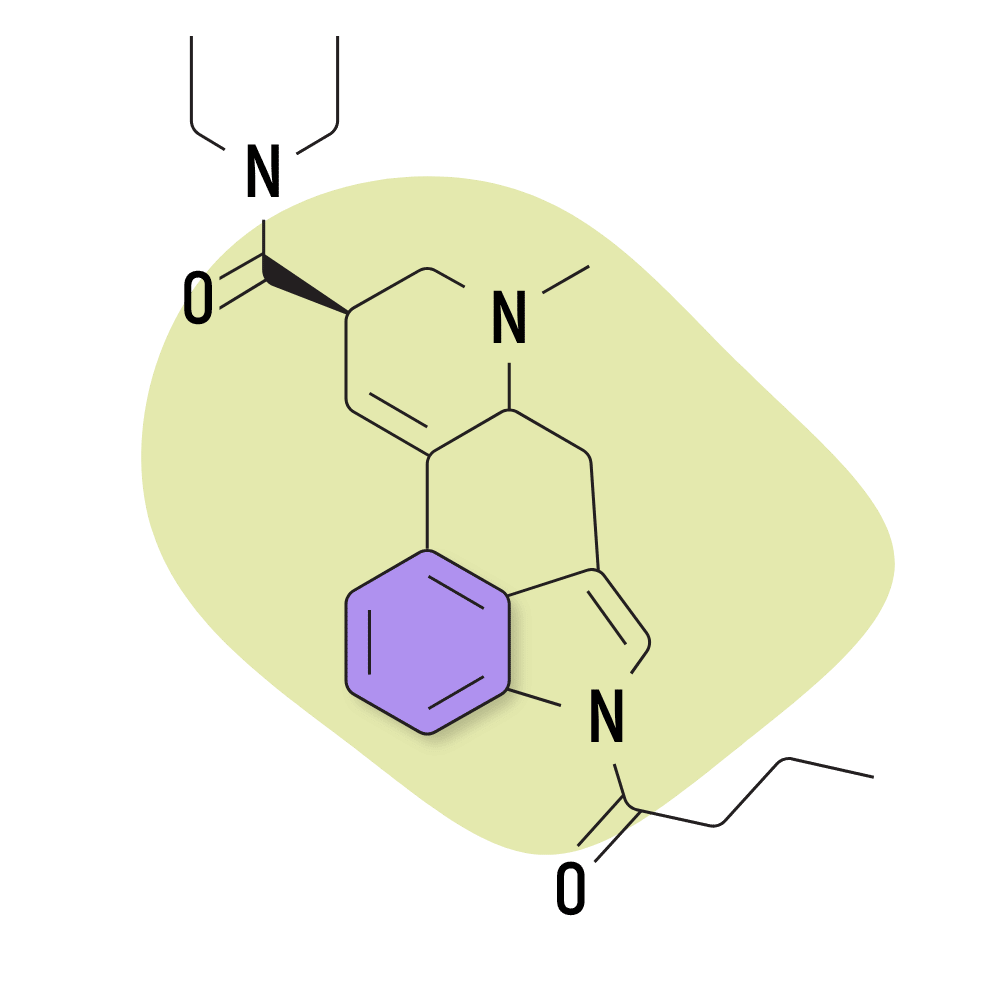
They feature a carboxamide group on the eighth carbon and can feature any number of chemical groups on the R1, RN1, RN2, and R6 positions. The effects may vary depending on which functional groups are attached. Some, such as ETH-LAD, appear to have a stronger potency than LSD, while others, such as 2-Bromo-LSD may actually have the opposite effect.
List of Common Lysergamides
There are dozens of lysergamides currently known and likely dozens more that haven’t yet been discovered. Here, we’ll cover the most common members of the class.
| Chemical Structure | Compound Name | Estimated Threshold Dose |
| 1B-LSD | 25 micrograms |
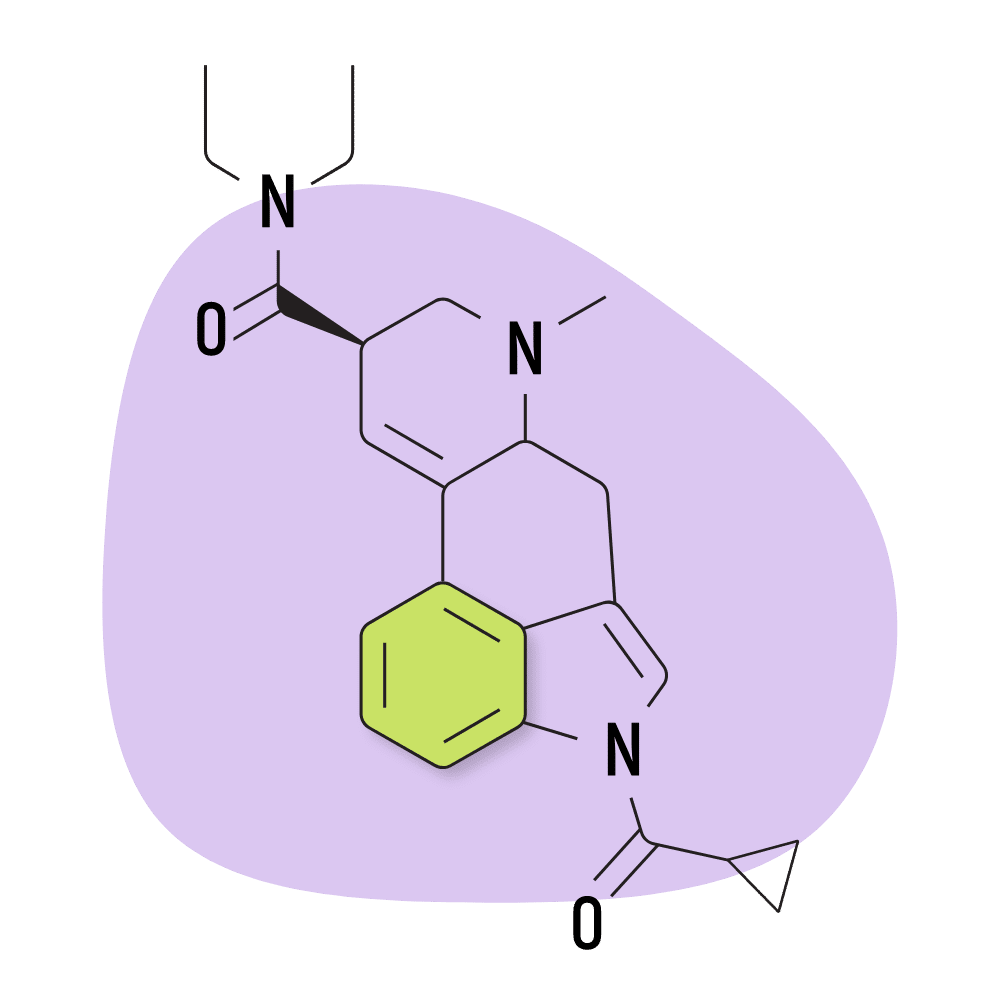
| 1cP-LSD | 25 micrograms |
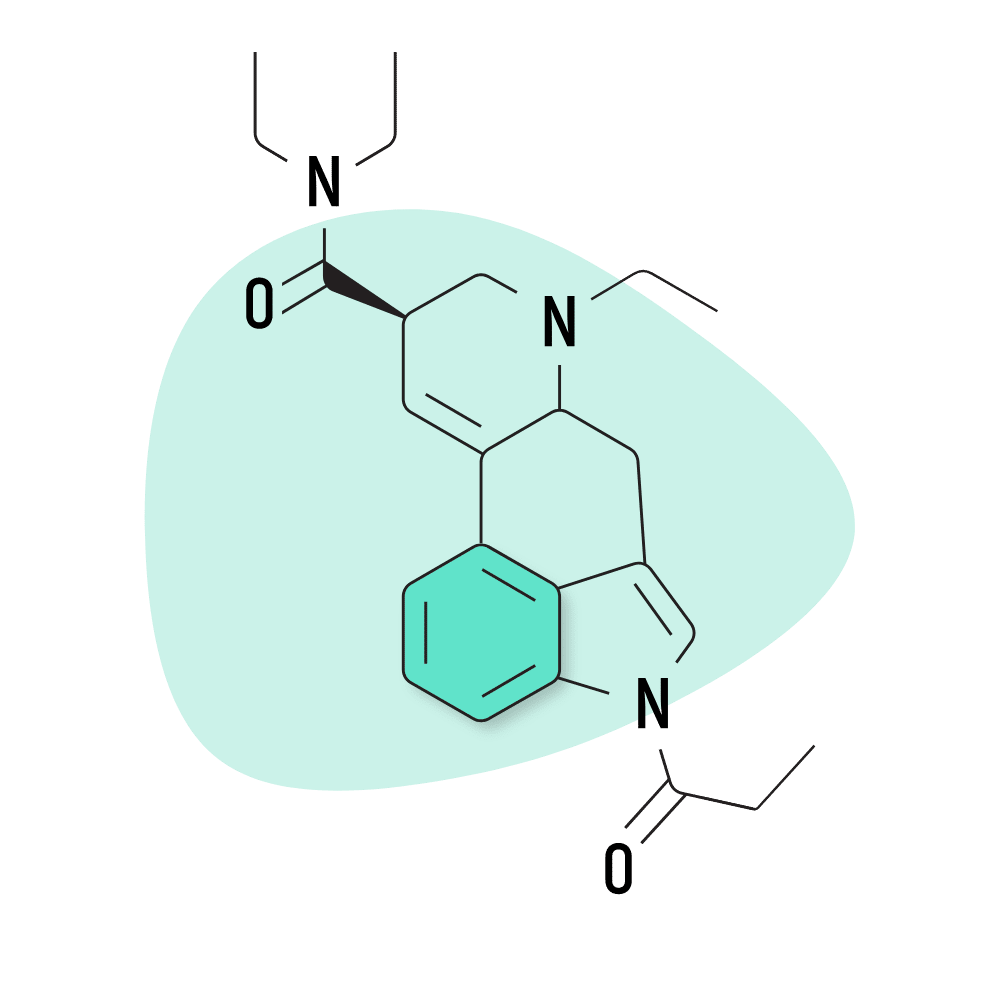
| 1P-ETH-LAD | 20 micrograms |
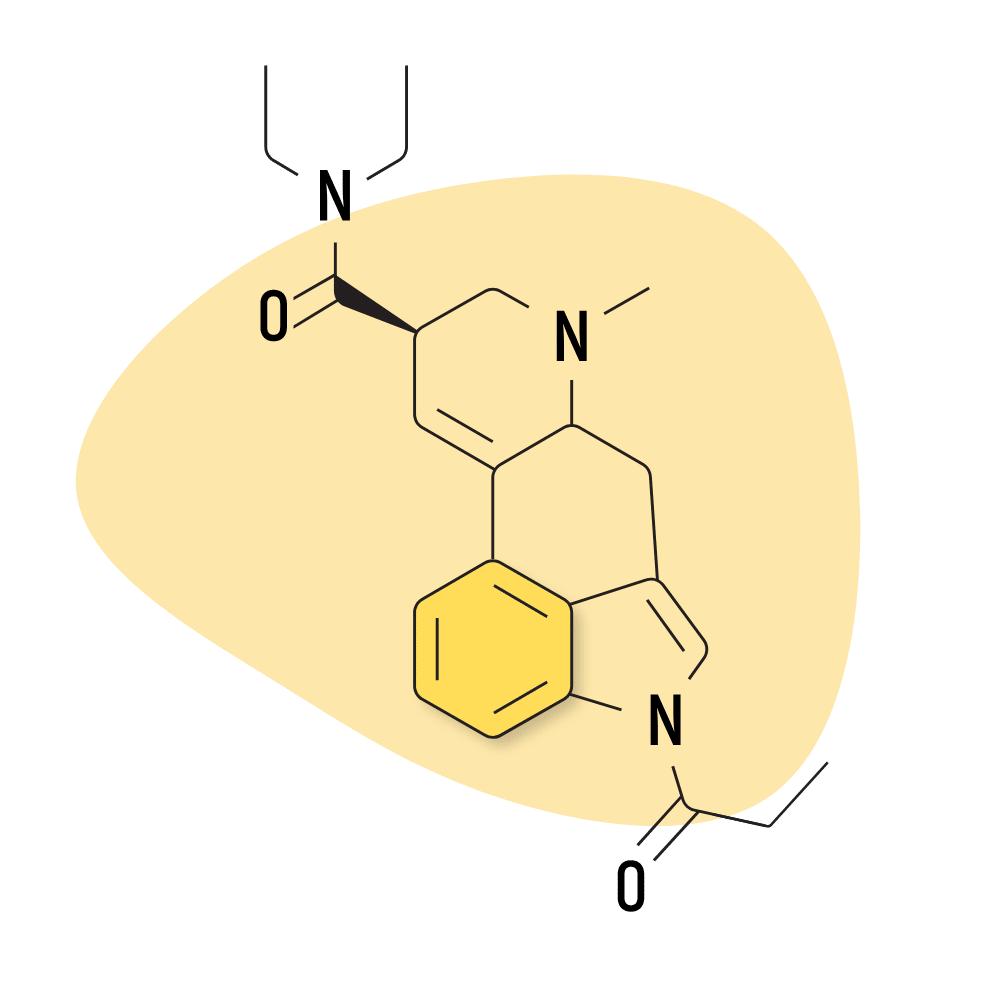
| 1P-LSD | 25 micrograms |
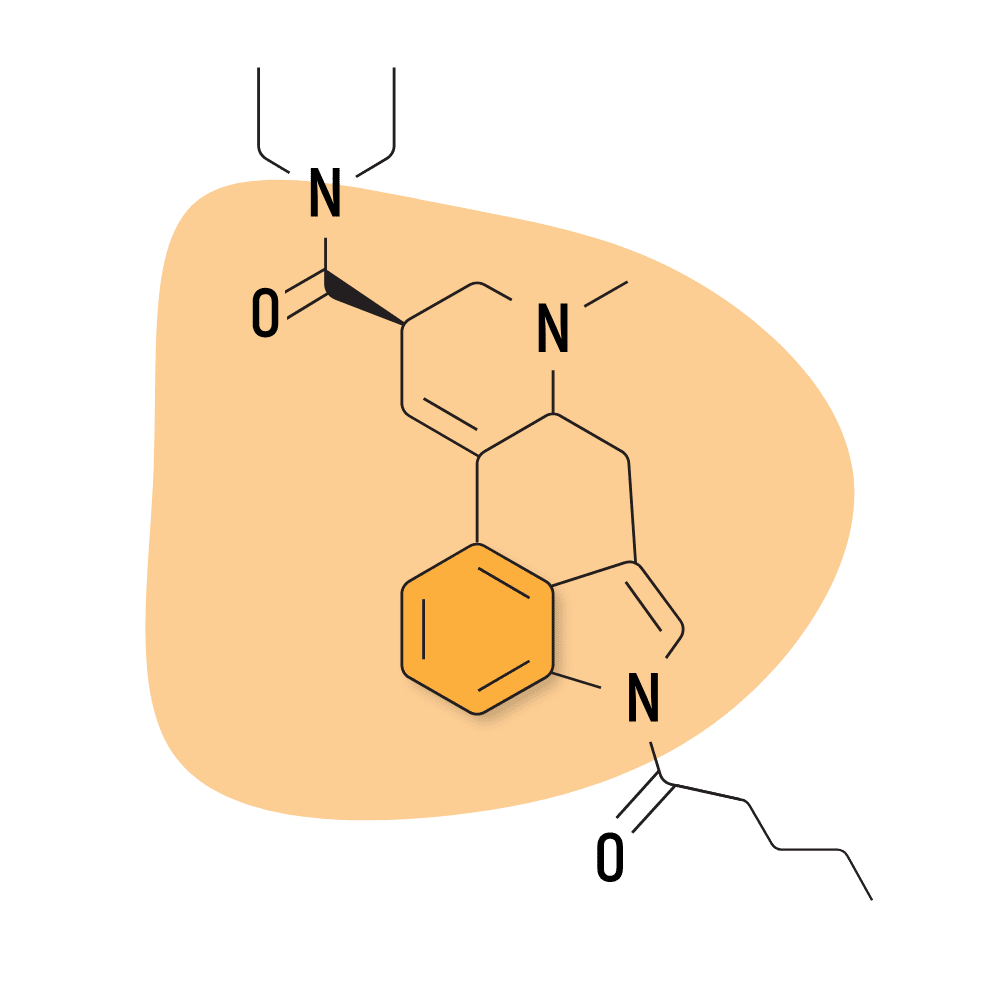
| 1V-LSD | 25 micrograms |
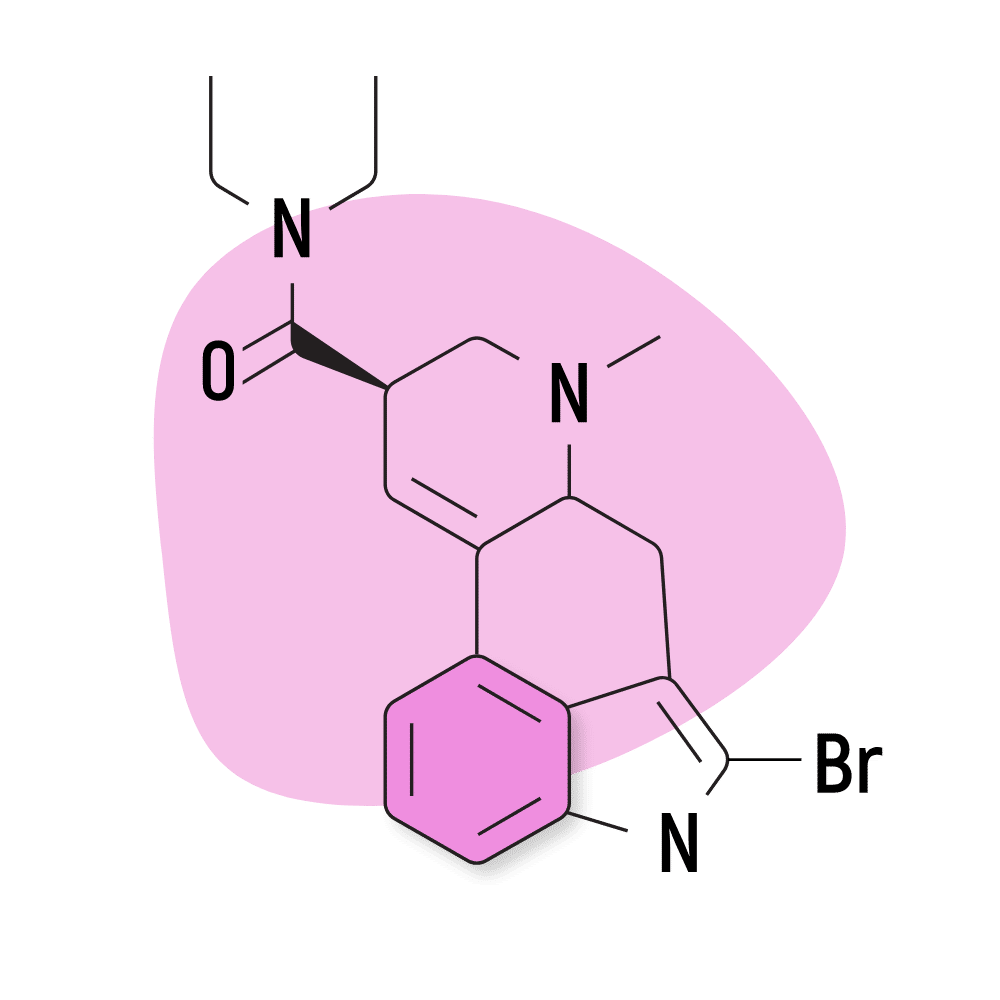
| 2-Bromo-LSD | Not Active |
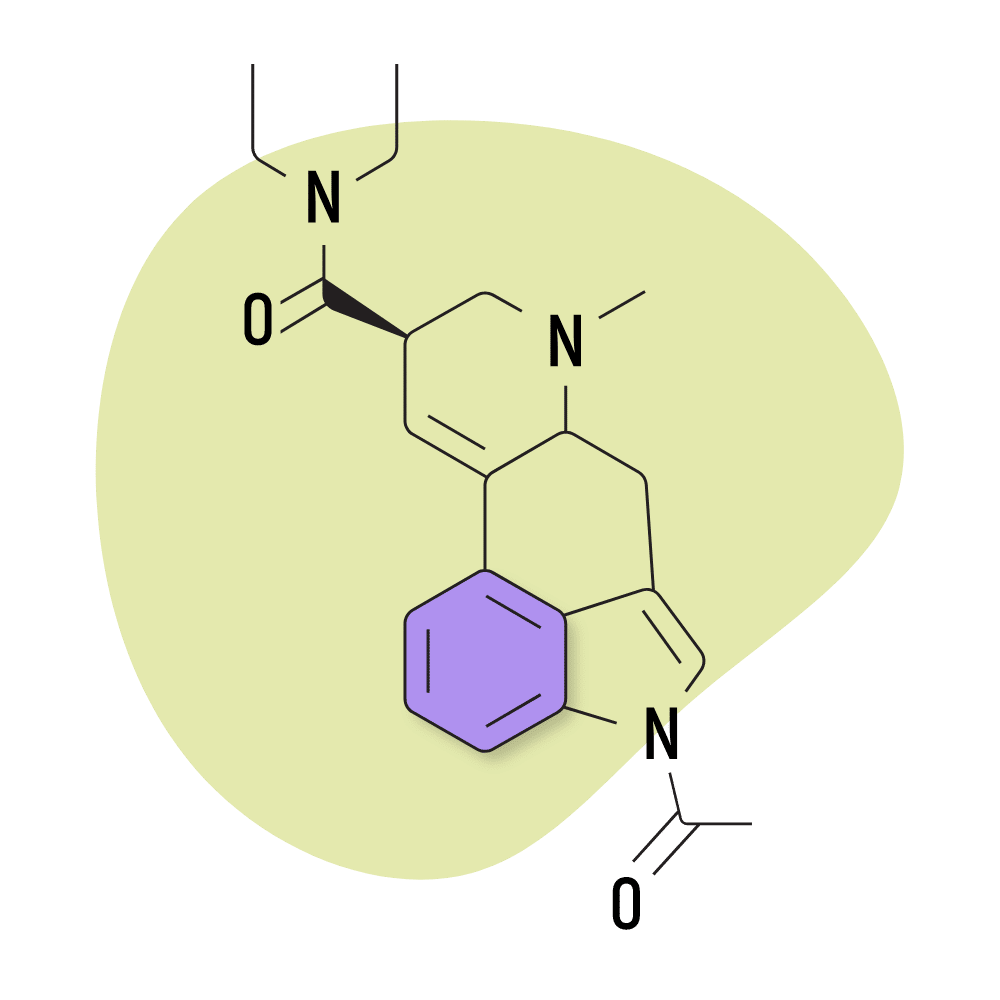
| AL-LAD | 40 micrograms |
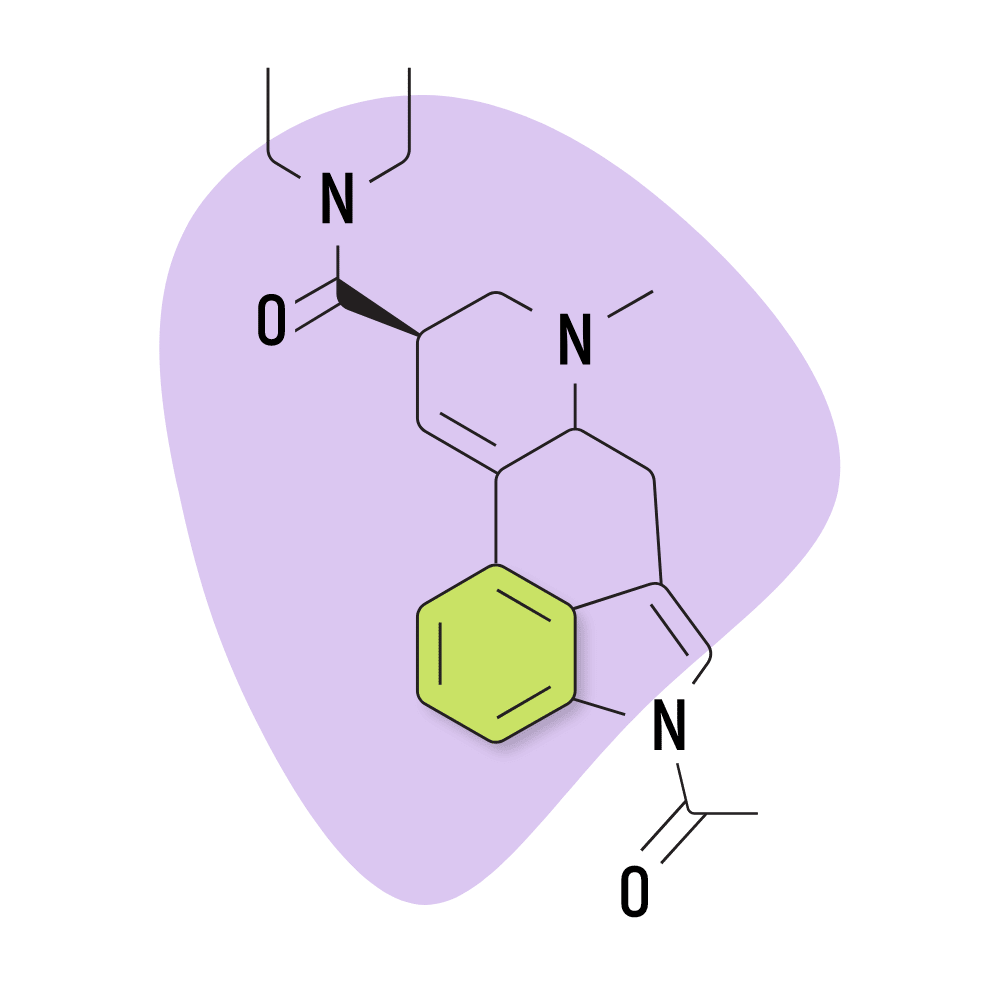
| ALD-52 | 25 micrograms |
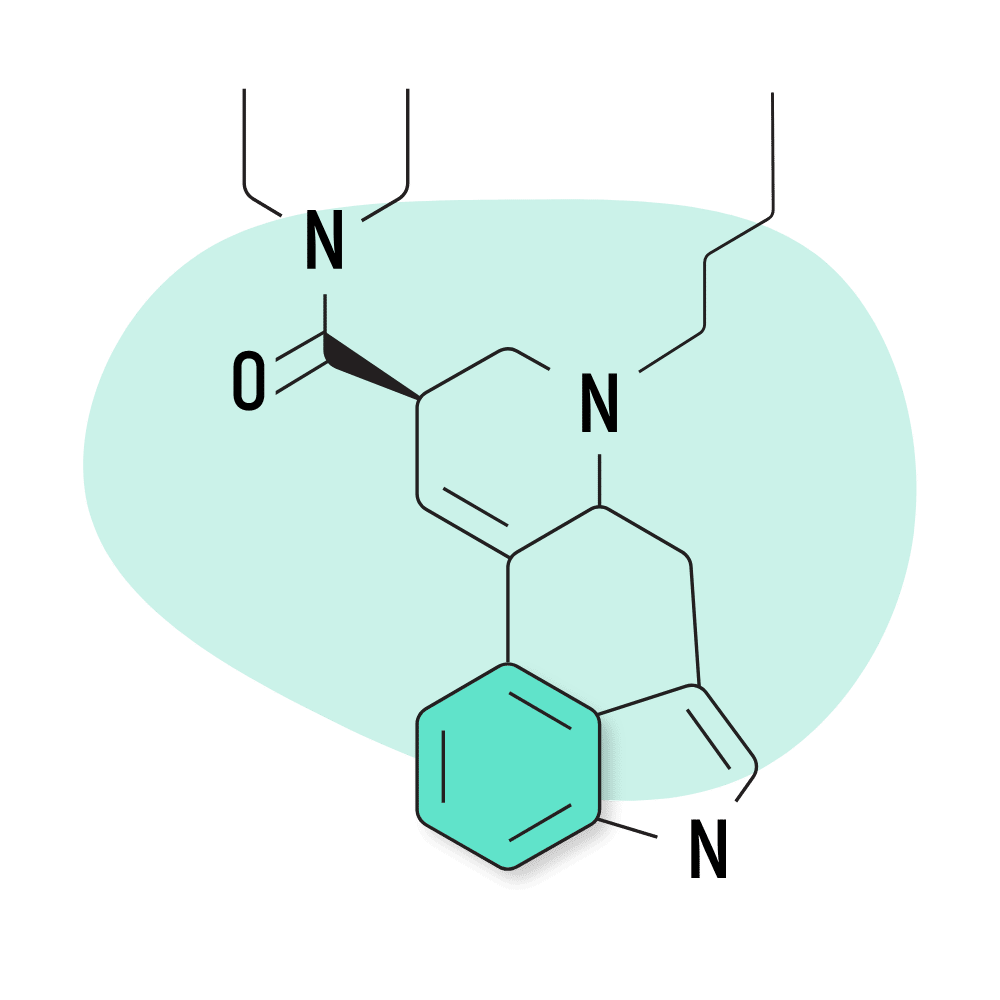
| BU-LAD | Unknown |
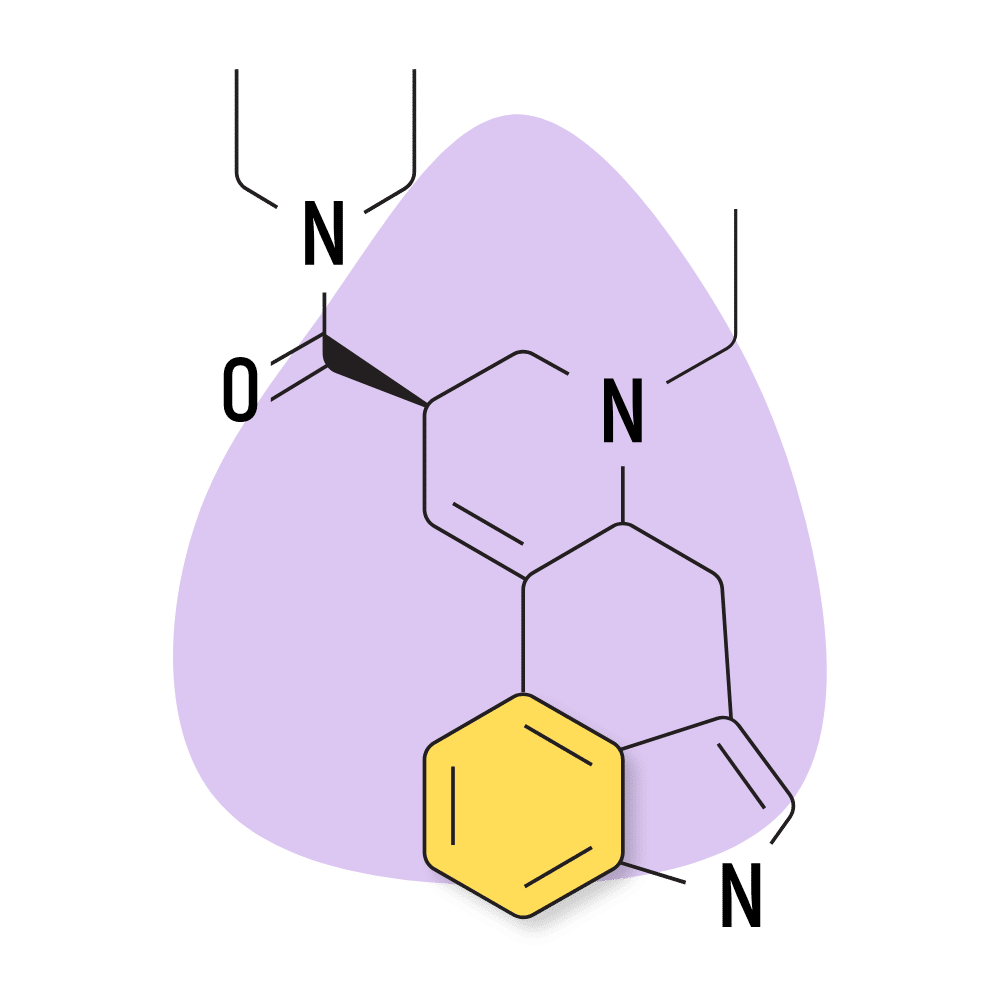
| ETH-LAD | 15 micrograms |
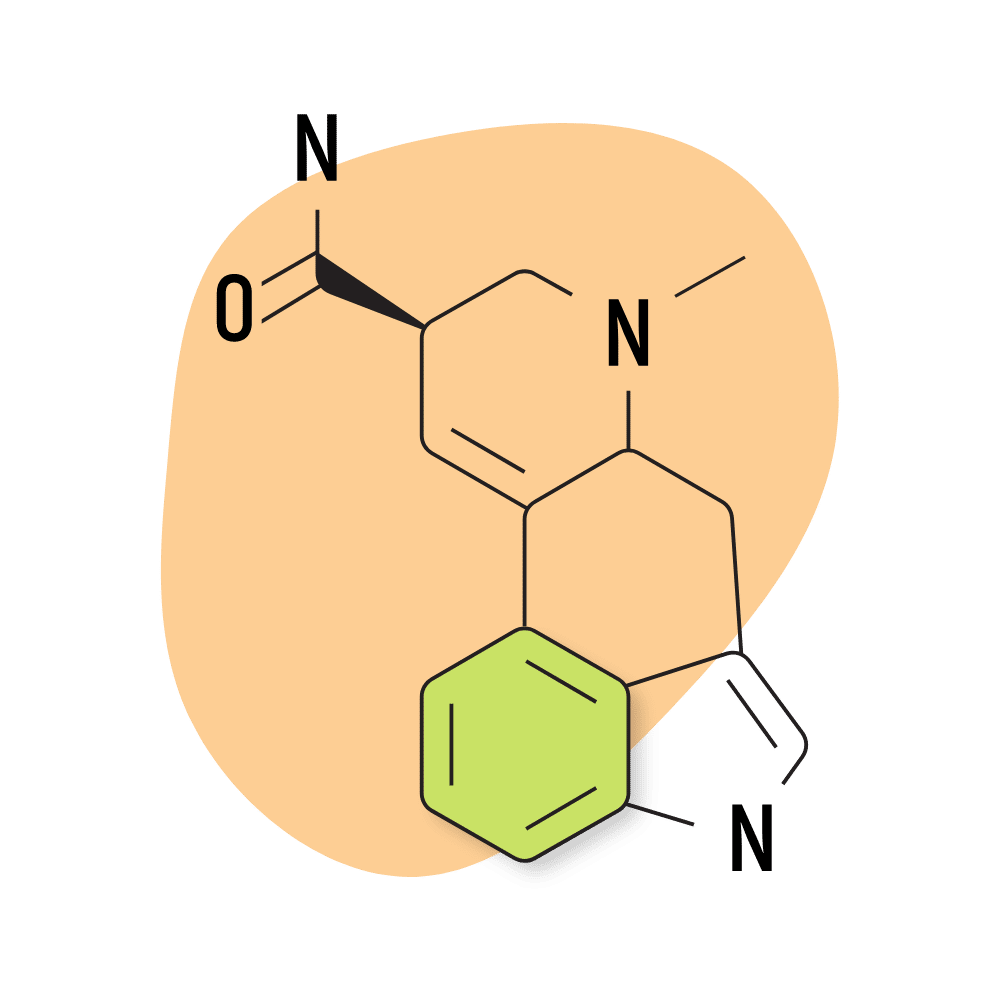
| LSA | 400 micrograms |
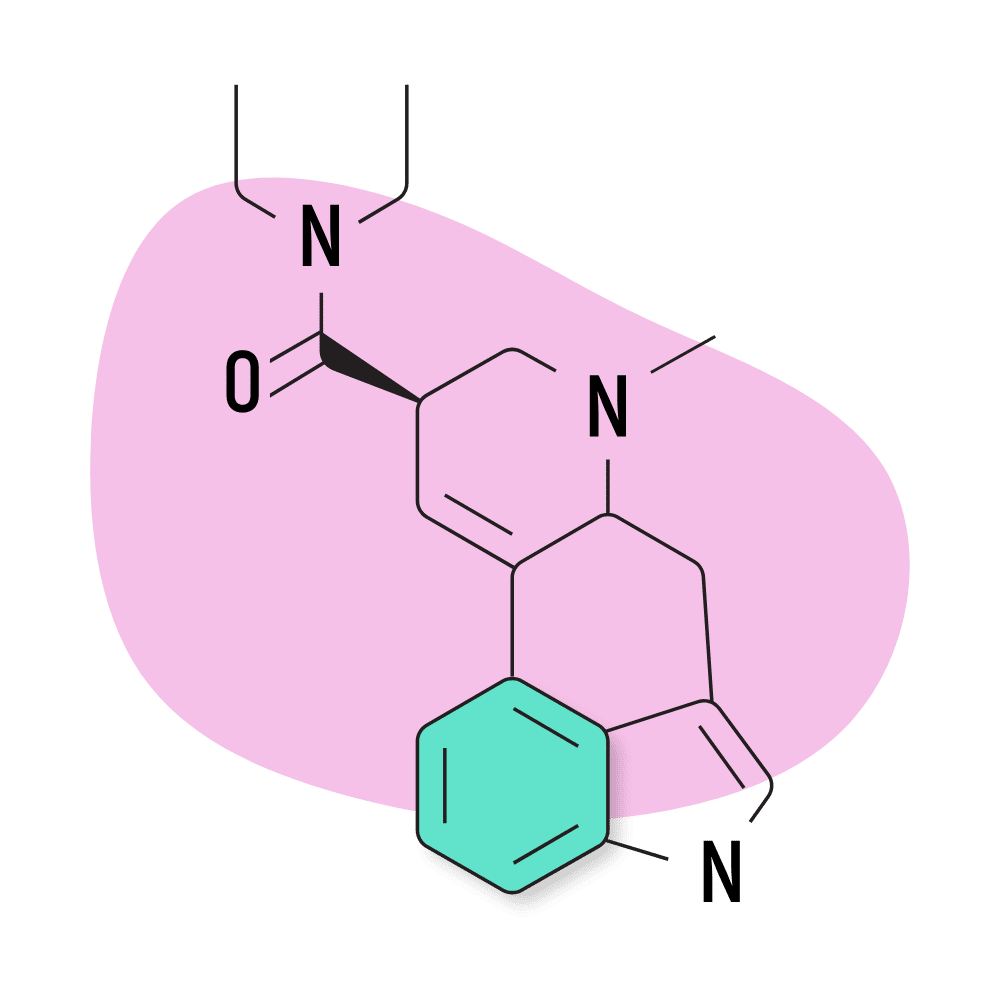
| LSD | 25 micrograms |
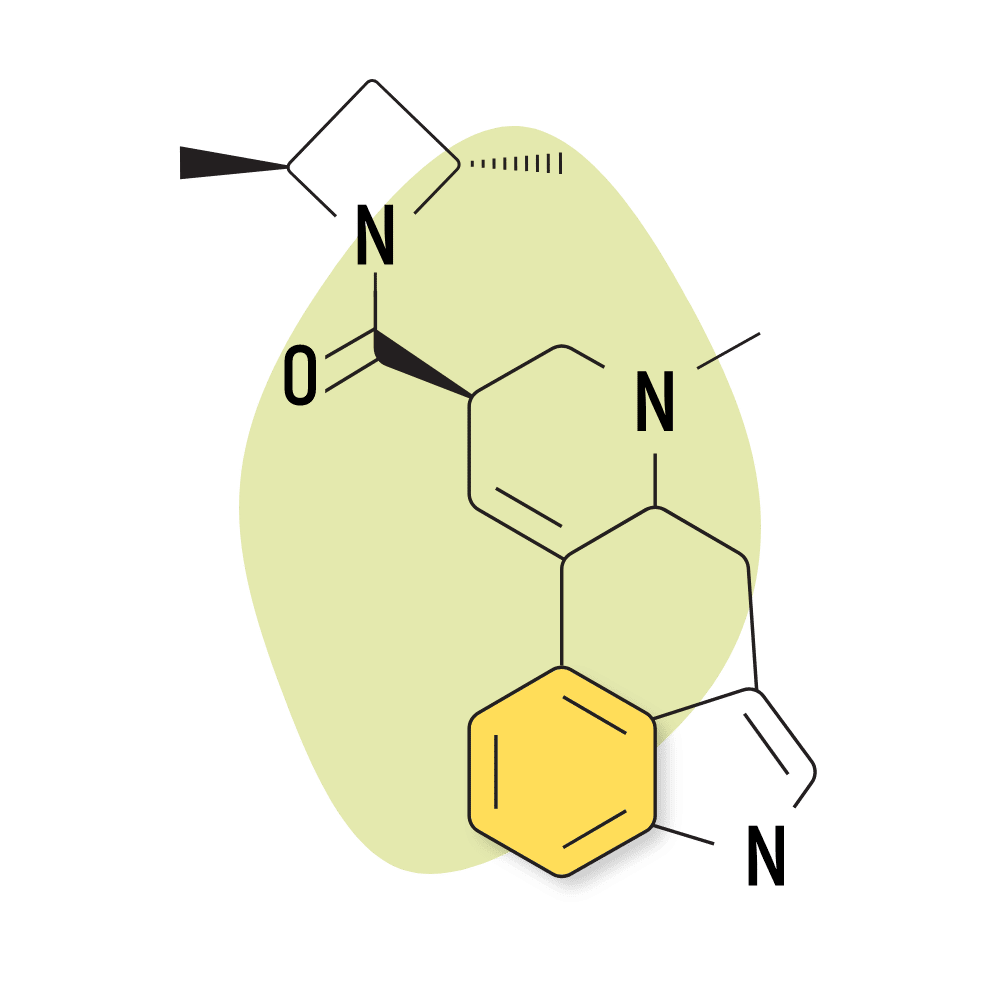
| LSZ | 20 micrograms |
| PARGY-LAD | 160 micrograms |
| PRO-LAD | 30 micrograms |
1. 1B-LSD
1B-LSD (1-butyryl-lysergic acid diethylamide) is an acetylated version of LSD. There isn’t much research available on this compound, but preliminary studies suggest it to be around 15% of the strength of LSD [5]. Aside from being less potent, the effects are practically indistinguishable from both LSD and 1P-LSD.
1B-LSD was popular in Europe and Canada for a while as a “legal acid,” but new laws have since been imposed to ban this research chemical.

1B-LSD Specs:
- Chemical Name: 1-butyryl-lysergic acid diethylamide
- IUPAC Name: (6aR,9R)-4-butanoyl-N,N-diethyl-7-methyl-6,6a,8,9-tetrahydroindolo[4,3-fg]quinoline-9-carboxamide
- Duration of Effects: 8–12 Hours
- Estimated Threshold Dose: 25 micrograms
2. 1cP-LSD
1cP-LSD (1-(cyclopropylmethanoyl)-lysergic acid diethylamide) is an acylated derivative of LSD with similar potency to 1P-LSD [6]. Similarly, 1cP-LSD is believed to be a prodrug for LSD.
Like many research chemicals, 1cP-LSD was created for the sole purpose of avoiding laws that banned known psychedelics. This particular compound first entered the market in Germany and the Netherlands in 2019, shortly after the government banned 1P-LSD.

1cP-LSD Specs:
- Chemical Name: 1-(cyclopropylmethanoyl)-lysergic acid diethylamide
- Duration of Effects: 8–12 Hours
- Estimated Threshold Dose: 25 micrograms
3. 1P-ETH-LAD
1P-ETH-LAD (1-Propionyl-6-ethyl-6-nor-lysergic acid diethylamide) is believed to serve as a prodrug for ETH-LAD — one of the stronger members of the lysergamide family. The experience is considered more visual than LSD and has less of an impact on emotion and mood.
This compound was made to avoid regulation but has since been banned in most European countries, Canada, the US, and Australia.

1P-ETH-LAD Specs:
- Chemical Name: 1-Propionyl-6-ethyl-6-nor-lysergic acid diethylamide
- Duration of Effects: 6–12 Hours
- Estimated Threshold Dose: 20 micrograms
4. 1P-LSD
1P-LSD (1-propionyl-lysergic acid diethylamide) is a modified version of LSD. It features a propionyl group attached to the nitrogen molecule in the indole portion of LSD. This makes the molecule inactive but is quickly metabolized by the liver into LSD.
Because of the prodrug status of 1P-LSD, the potency tends to vary for each user and has a slower onset time than LSD. For most people, the subjective effects are on-par with LSD.
This was one of the first research chemicals developed to provide “legal LSD” to the market sometime around 2012. Most countries have since banned the substance or enacted analog laws that ban psychoactive isomers of banned substances by default. Currently, 1P-LSD is believed to be legal in Canada.

1P-LSD Specs:
- Chemical Name: 1-propionyl-lysergic acid diethylamide
- Duration of Effects: 8–12 Hours
- Estimated Threshold Dose: 25 micrograms
5. 1V-LSD
1V-LSD (1-Valeroyl-d-lysergic acid diethylamide) — sometimes referred to as Valerie — is a modified version of 1P-LSD. While there’s virtually no research available on this compound, it’s believed to act as a prodrug for LSD and exhibits near-identical effects.
This compound is the latest invention in a series of chemicals invented in response to various bans in Europe. First, LSD was banned, so analogs like 1P-LSD were invented. Then 1P-LSD was banned, so 1cP-LSD was made. After 1cP-LSD was banned too, 1V-LSD was designed to take its place. This game of cat and mouse continues to this day.
Places with analog laws, like the US, UK, and Austria, consider 1V-LSD explicitly illegal because it’s a psychoactive derivative of LSD. Switzerland recently added the substance to its prohibited substance list, but the compound remains in a legal grey area throughout most of Europe, Canada, and Australia today.

1V-LSD Specs:
- Chemical Name: 1-Valeroyl-d-lysergic acid diethylamide
- Duration of Effects: 8–12 Hours
- Estimated Threshold Dose: 25 micrograms
6. 2-Bromo-LSD
2-Bromo-LSD (2-Bromolysergic acid diethylamide) — sometimes called BOL-148 — is believed to block the effects of LSD. It binds to the same receptors but does not exert any psychedelic activity.
This compound was one of many ergotamine derivatives invented by Albert Hofmann and his team in the late 1950s. The goal of creating these compounds was to find a new treatment for migraine headaches. This eventually led to the accidental discovery of the lysergamide psychedelics.
2-Bromo-LSD has been shown to offer some support for cluster headache attacks, and due to the lack of psychoactivity compared to LSD, it may prove useful for this application in the near future. However, more research is needed to confirm the safety and efficacy of this compound for this application.
It’s also possible this compound could be used to dull the effects of LSD [7].

2-Bromo-LSD Specs:
- Chemical Name: 2-Bromolysergic acid diethylamide
- Duration of Effects: Unknown
- Estimated Threshold Dose: Not Active
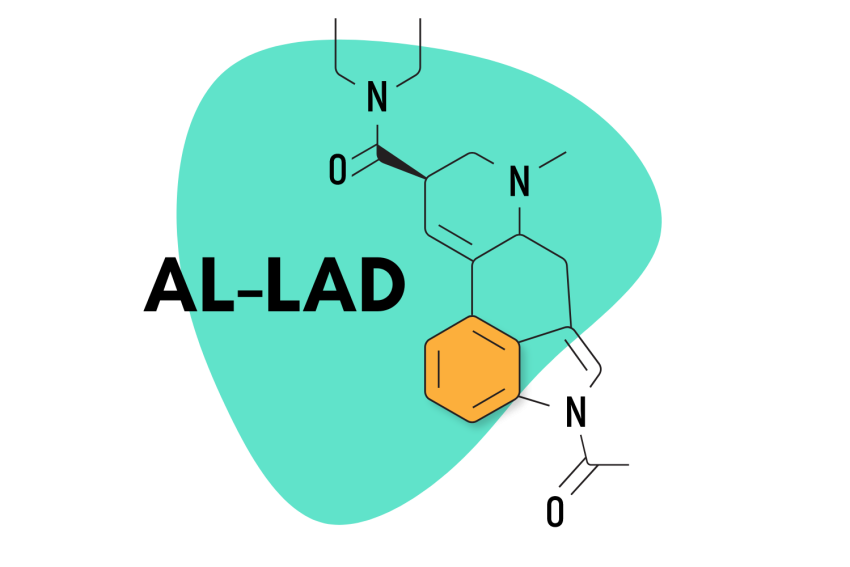
7. AL-LAD
AL-LAD (N6-allyl-6-norlysergic acid diethylamide) is one of the more common lysergamide derivatives. Compared to LSD, AL-LAD is favored for its slightly milder but more visual effects. It also tends to be less introspective and more “recreational” than LSD — which can often lead users to feel deeply introspective and philosophical.
This compound isn’t new. It’s been around since the 70s and has been formally studied several times already. Dr. David E. Nichols and his team at Purdue University have focused on this chemical in at least one study, and the compound earned a mention in Alexander Shulgin’s book, TIHKAL.

AL-LAD Specs:
- Chemical Name: 6-allyl-6-norlysergic acid diethylamide
- Duration of Effects: 7–11 Hours
- Estimated Threshold Dose: 40 micrograms
8. ALD-52
ALD-52 (1-acetyl-N,N-diethyllysergamide) is a homolog of 1P-LSD — the two are identical aside from 1P-LSD containing one additional carbon atom.
Unsurprisingly, the effects of ALD-52 and 1P-LSD are virtually identical. ALD-52 serves as a prodrug for LSD, only becoming active after it’s processed by the liver. This can delay the onset of effects by as much as one additional hour compared to LSD — though most people start to experience the effects within about 60 minutes of taking it.
This lysergamide was first synthesized in Hofmann’s lab in the 1950s. It was believed to be the active ingredient in a particularly strong batch of LSD called Orange Sunshine (produced by Nick Sand), but this was later debunked by Sand himself. The effects of ALD-52 are considered equivalent, if not slightly weaker than LSD.

ALD-52 Specs:
- Chemical Name: 1-acetyl-N,N-diethyl-lysergamide
- Duration of Effects: 7-11 Hours
- Estimated Threshold Dose: 25 micrograms
9. BU-LAD
BU-LAD (6-butyl-6-nor-lysergic acid diethylamide) was one of several lysergamide compounds invented by Alexander Shulgin. It was published in his book, TiHKAL, but hasn’t received much attention since this because of its weak effect profile. Shulgin reportedly consumed a dose of 500 micrograms of BU-LAD and reported only mild psychoactive effects.

BU-LAD Specs:
- Chemical Name: 6-butyl-6-nor-lysergic acid diethylamide
- Duration of Effects: Unknown
- Estimated Threshold Dose: Unknown
10. ETH-LAD
ETH-LAD (6-ethyl-6-nor-lysergic acid diethylamide) is one of the most popular lysergamides aside from LSD. Its popularity is likely the result of its slightly stronger effect profile. The threshold dose of this compound is only 20 micrograms. For comparison, the threshold of LSD is closer to 30 micrograms.
Despite its popularity, there’s limited information regarding safety or qualitative effect differences.
The drug creator, Alexander Shulgin, claims ETH-LAD produces strong visual effects but is less likely to produce unwanted physical sensations compared to tryptamine psychedelics.

ETH-LAD Specs:
- Chemical Name: 6-ethyl-6-nor-lysergic acid diethylamide
- Duration of Effects: 8–12 Hours
- Estimated Threshold Dose: 15 micrograms
11. LSA
LSA (d-lysergic acid amide) is a naturally-occurring lysergamide found in the ergot fungus as well as the seeds of morning glory and Hawaiian baby woodrose (both from the Convulaceae family).
LSA was discovered in 1932 and classified under the name “ergine” [8]. Other sources use the term LAE-111.
Compared to other lysergamides, LSA is much less potent and has a distinct sedative action. This combination gives LSA a distinct oneriogenic effect that’s absent from most of the other compounds in this category.
The conventional dose of LSA is around 500 micrograms to 1 gram, making it less than 10% as potent as LSD. Most LSA is derived from either morning glory or Hawaiian baby woodrose seeds in the form of tinctures, but isolated LSA is also available.
The effects of LSA are described as lucid and dreamy but come with some negative qualities as well. The body load from LSA can be uncomfortable and, in some cases, extreme, producing feelings of nausea, stomach cramping, tingling, and dizziness. These effects can be quite uncomfortable.

LSA Specs:
- Chemical Name: (6aR,9R)-7-methyl-6,6a,8,9-tetrahydro-4H-indolo[4,3-fg]quinoline-9-carboxamide
- Duration of Effects: 6–12 Hours
- Estimated Threshold Dose: 300 micrograms
12. LSD
LSD (lysergic acid diethylamide) — AKA Lucy, LAD, and Acid — is hands down the most popular member of the lysergamide family of psychedelics. While LSD isn’t necessarily the strongest, it was the first to be explored scientifically. It provides long-lasting and profound effects while lacking any major safety risks.
Part of the reason this compound became so popular is because of advocates like Timothy Leary, Leo Zeff, Richard Alpert, and others throughout the 1960s and 70s. LSD was supplied to Leary, Alpert, and the rest of their team by Sandoz Pharmaceuticals as part of the Harvard Psilocybin Project. At the time, LSD was perfectly legal, and Sandoz wanted researchers to conduct research on their new compound in order to find a potential use for it.
After LSD became a symbol for the 60s counterculture and hippie movement, then-president Ronald Regan banned the substance and declared war on drugs. This, of course, brought all research on LSD and other psychedelics to a halt for several decades.
The US government had a secret project called MK-ULTRA in the 60s, 70s, and 80s as well, which attempted to use LSD for more nefarious purposes like mind control and truth serums.

LSD Specs:
- Chemical Name: D-lysergic acid diethylamide
- IUPAC Name: (6aR,9R)-N,N-diethyl-7-methyl-6,6a,8,9-tetrahydro-4H-indolo[4,3-fg]quinoline-9-carboxamide
- Duration of Effects: 8–12 Hours
- Estimated Threshold Dose: 25 micrograms
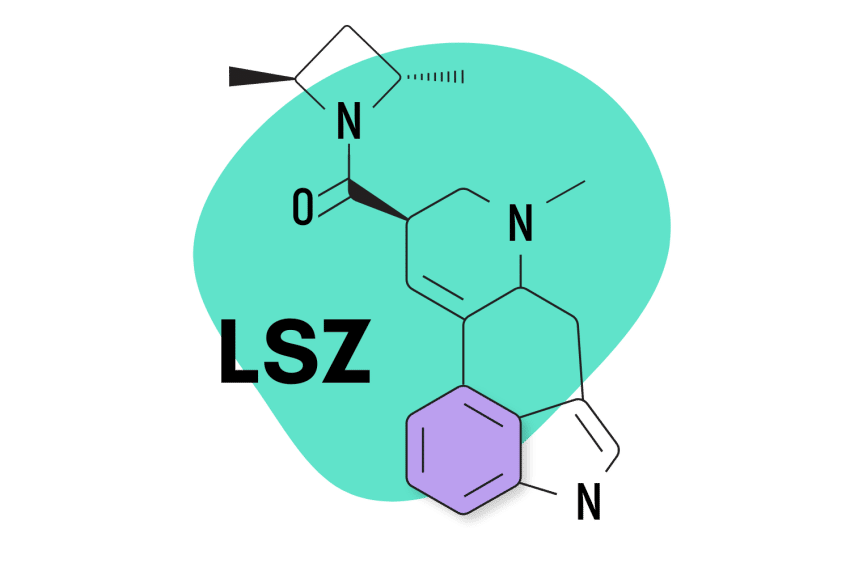
13. LSZ
LSZ (lysergic acid 2,4-dimethylazetidide) was first discovered by David E. Nichols at Purdue University in the 1980s. The effects are virtually identical to LSD but with slightly higher potency and longer-lasting effects. The come-up is also slower, taking up to 1.5 hours to begin feeling effects.
Like many lysergamides, there isn’t much research on this compound, but so far, there’s no indication this compound poses any significant health risks.
Anecdotal reports suggest this compound has a higher emphasis on the emotional experiences of lysergamide psychedelics, less visual intensity, and a somewhat sedative nature in higher doses. Many people claim this to be the best psychedelic for use in the evenings because it’s easier to fall asleep afterward.

LSZ Specs:
- Chemical Name: Lysergic acid 2,4-dimethylazetidide
- IUPAC Name: [(6aR,9R)-7-methyl-6,6a,8,9-tetrahydro-4H-indolo[4,3-fg]quinolin-9-yl]-[(2S,4S)-2,4-dimethylazetidin-1-yl]methanone;(2R,3R)-2,3-dihydroxybutanedioic acid
- Duration of Effects: 6–12 Hours
- Estimated Threshold Dose: 20 micrograms
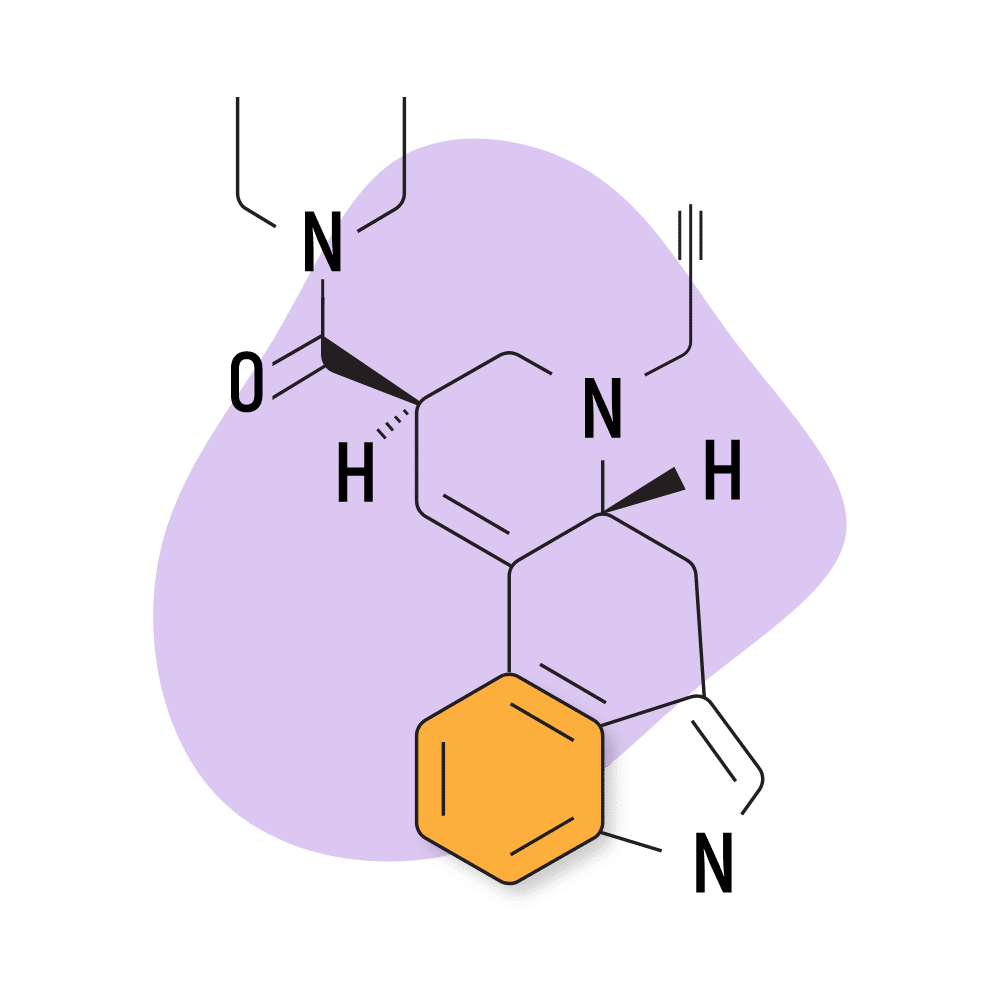
14. PARGY-LAD
PARGY-LAD (6-propynyl-6-nor-lysergic acid diethylamide) is a homolog of LSD with a similar effect profile. The potency of this compound is reportedly much lower than LSD, however — with the threshold dose around 160 micrograms. According to Alexander Shulgin, the psychoactive dose for this compound is somewhere in the ballpark of 500 micrograms — making this compound roughly 40% as strong as LSD.
This compound was included in Shulgin’s TiHKAL, but not much has been published in the literature since then. This is likely due to the lower potency combined with the challenges of manufacturing. This molecule is very similar to LSD and requires virtually the same synthesis process. It makes more sense to create the stronger LSD than the weaker PARGY-LAD.

PARGY-LAD Specs:
- Chemical Name: 6-propynyl-6-nor-lysergic acid diethylamide
- IUPAC Name: (6aR,9R)-N,N-diethyl-7-prop-2-ynyl-6,6a,8,9-tetrahydro-4H-indolo[4,3-fg]quinoline-9-carboxamide
- Duration of Effects: 6–8 Hours
- Estimated Threshold Dose: 160 micrograms
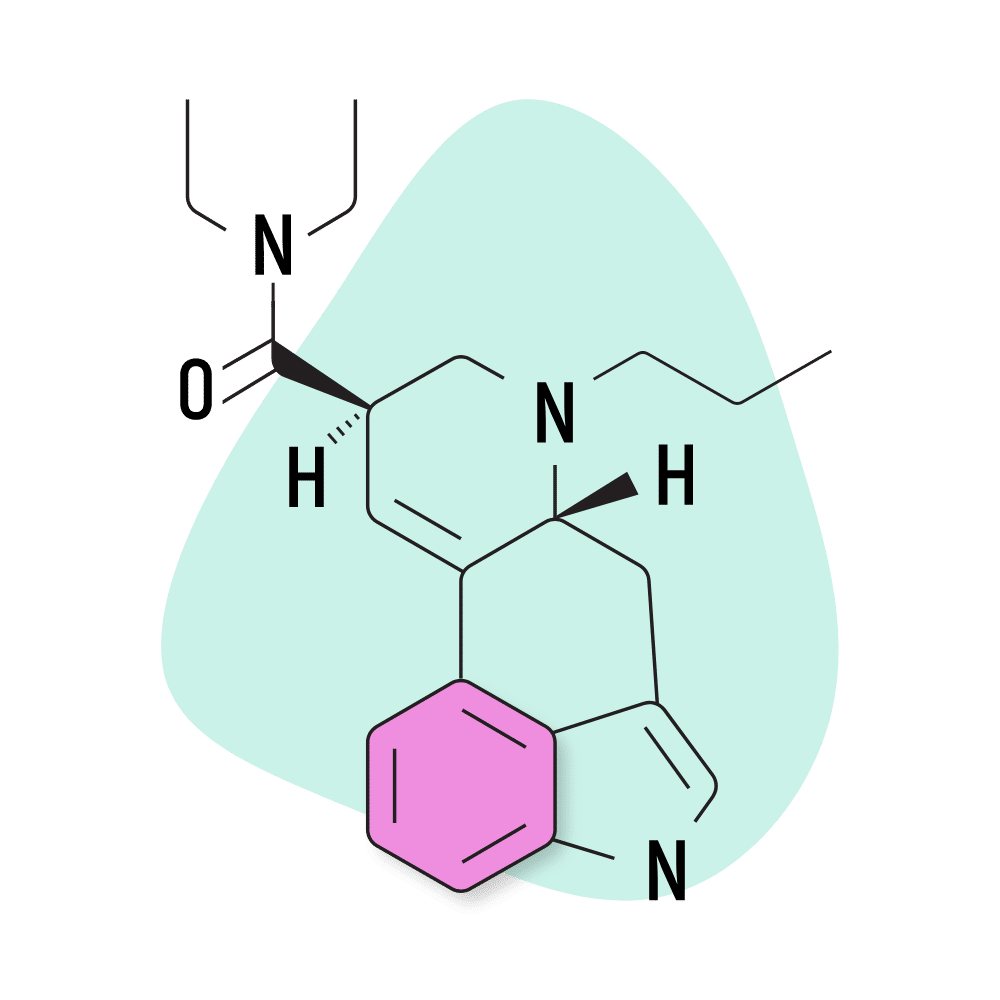
15. PRO-LAD
PRO-LAD (6-propyl-6-nor-lysergic acid diethylamide) is another creation of the Purdue University team led by David E. Nichols in the 1980s. This compound shares the same structure as LSD, with the addition of just two extra carbons. This changes the potency and effect profile slightly — though most people probably wouldn’t be able to tell the difference.
Some users suggest PRO-LAD has a stronger body load than LSD — producing sensations like tingling in the fingers and toes, a sensation that one is either floating or sinking into the ground, and increased awareness of other bodily sensations.

PRO-LAD Specs:
- Chemical Name: 6-propyl-6-nor-lysergic acid diethylamide
- IUPAC Name: (6aR,9R)-N,N-diethyl-7-propyl-6,6a,8,9-tetrahydro-4H-indolo[4,3-fg]quinoline-9-carboxamide
- Duration of Effects: 6–8 Hours
- Estimated Threshold Dose: 30 micrograms
16. Other Lysergamides
The list doesn’t end here. The compounds listed above only cover the lysergamides that have been tested in some way for effects, potency, and safety. There are likely hundreds of undiscovered and untested lysergamides. More research is needed to explore these compounds in more detail. It’s an exciting time to be a researcher at the moment. Any of the below compounds could potentially provide new, distinct effect profiles.
- Amesergide
- CYP-LAD
- DAL
- DAM-57
- ECPLA
- EIPLA
- ETFELA
- IP-LAD
- iso-LSD
- LAcB
- LAE-32
- LAiP
- LAtB
- LPD-824
- LSB
- LSD-Pip
- LSH
- LSM-775
- LSP
- LY-215840
- Methergine
- Methysergide
- MiPLA
- MLD-41
- MPLA
Ergot Alkaloids
LSD and the rest of the lysergamide family are based on the structure of ergot alkaloids such as ergoline.
This group of naturally-occurring alkaloids is produced in several species of Claviceps fungi — including Claviceps purpurea, C. fusiformis, C. paspali, C.africana, C. lutea, and others. These fungi infect grains like wheat, rye, barley, and oats.
Each of these fungi produces large quantities of alkaloids such as ergometrine, ergotamine, and about a dozen others. The most common is ergotamine — accounting for roughly 2% of the dried weight of Claviceps spp.
Many of the ergot alkaloids are psychoactive but also cause an often fatal condition called ergotism (AKA “St. Anthony’s Fire”).
This condition causes severe burning sensations in the limbs, muscle spasms, fevers, hallucinations and delusions, paralysis, seizures, and death. There are reports of people entering a permanent daze or mania after consuming grains infected by the ergot fungus. Death often results from severe damage to the vascular system in which blood is no longer able to flow — leading to severe infection.
Lower doses of ergot alkaloids are sometimes used for treating vascular disorders, stimulating uterine contractions, and treating migraine or cluster headaches.
Several ergot alkaloids were put under the lens of the German pharmaceutical company Sandoz in the early 1900s, which eventually led to the creation of LSD in 1938.
1. Ergoline
Ergoline is the most basic of the ergot alkaloids and serves as the skeleton for virtually all other ergot alkaloids, as well as the synthetic and semi-synthetic class of the lysergamide psychedelics.
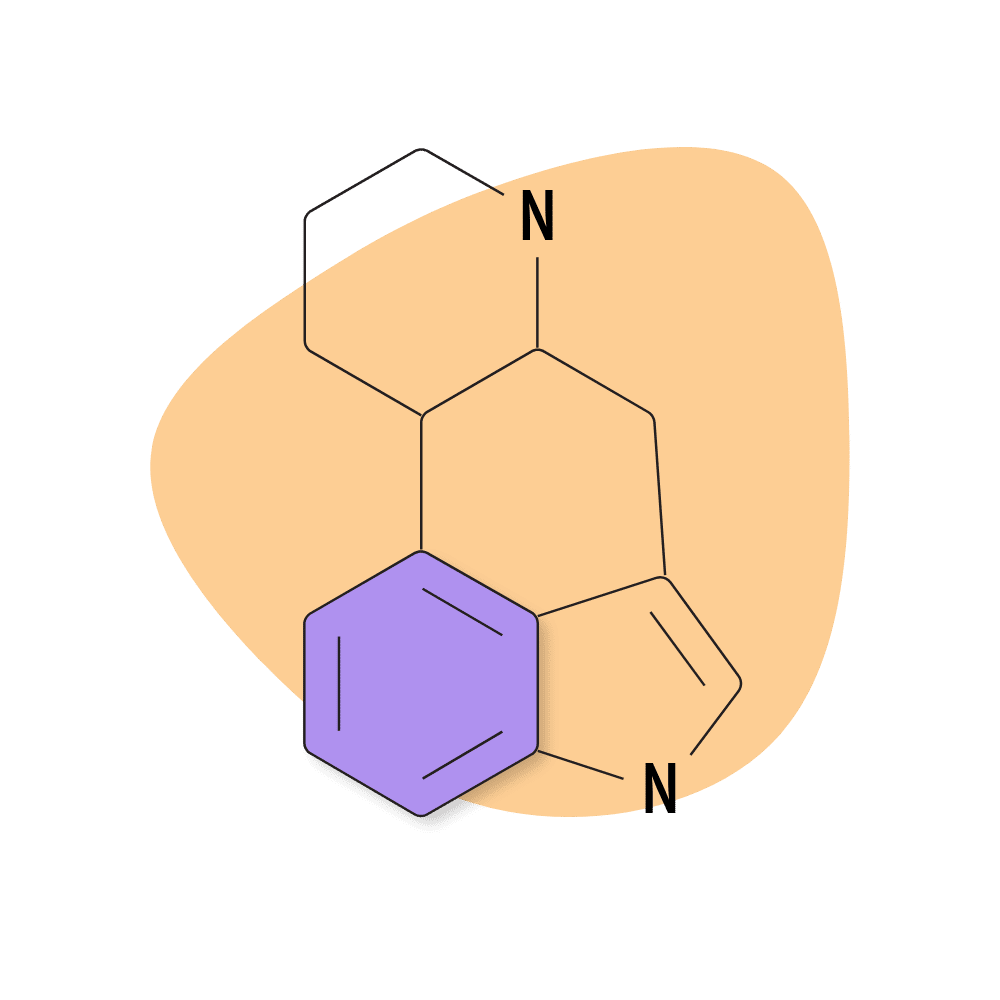
Ergoline itself is thought to have low psychoactive effects on its own. The addition of an amide group significantly enhances its ability to bind to the serotonin and dopamine receptors.
2. Ergometrine
Ergometrine was first discovered in 1932. It’s a naturally-occurring alkaloid from the ergot fungus. Albert Hofmann used this compound and several others as the starting point for synthesizing more advanced lysergamides like LSD and ALD-52.
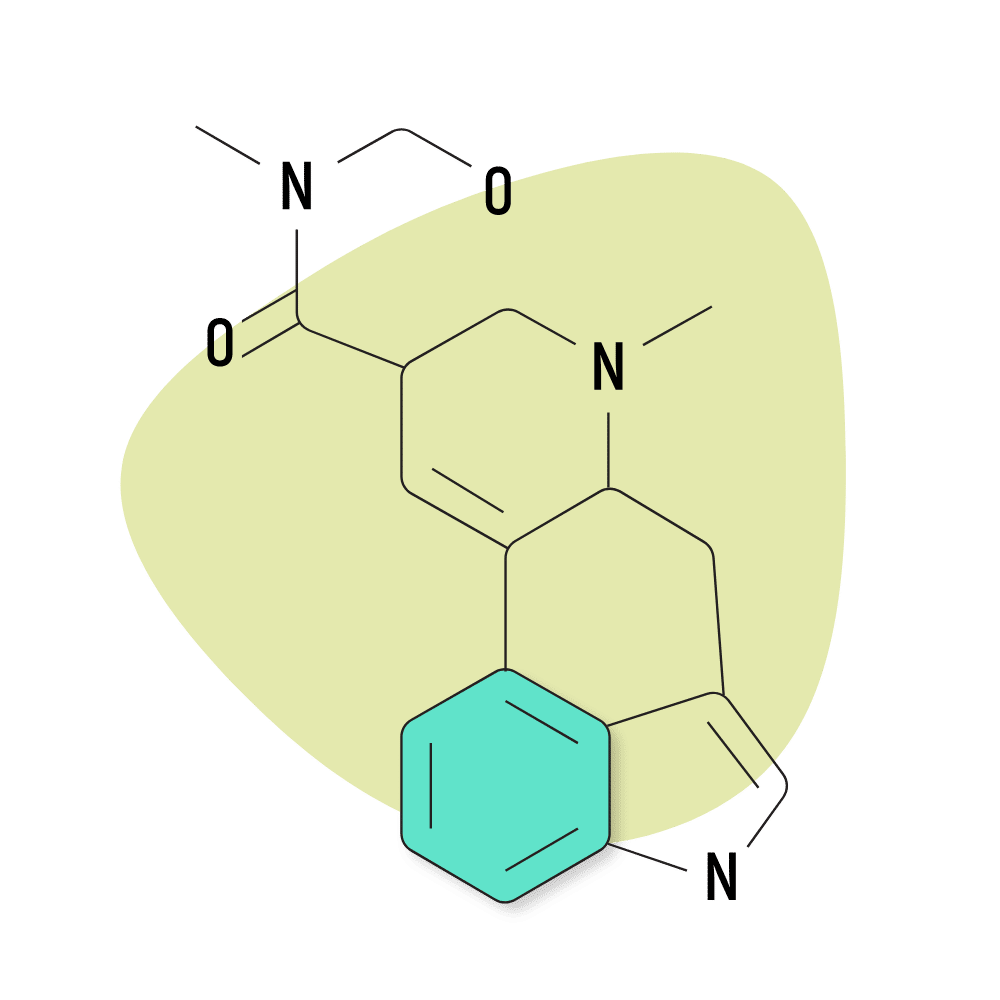
Ergometrine is reportedly psychedelic at doses over 2 mg. However, due to the potential for serious side effects, this compound is rarely used for this effect. Instead, it’s used as a medicine at roughly 10% of the psychoactive dose (0.2 mg) for treating hemorrhages during childbirth. It’s also used to facilitate the delivery of the placenta. For this effect, ergometrine is included on the WHO list of essential medicines.
Despite not being used directly as a psychedelic, this compound is highly regulated because it serves as a precursor for making LSD.
Ergometrine is sold under the brand names Ergotrate, Ergostat, and Syntometrine.
3. Ergotamine
Ergotamine is a naturally-occurring alkaloid from the ergot fungus, first discovered in 1918 by researchers at the Sandoz pharmaceutical company in Germany. It’s sometimes used as a treatment for migraine and cluster headache attacks in low doses. It’s sold under brand names like Cafergot (ergotamine and caffeine) and Ergomar.
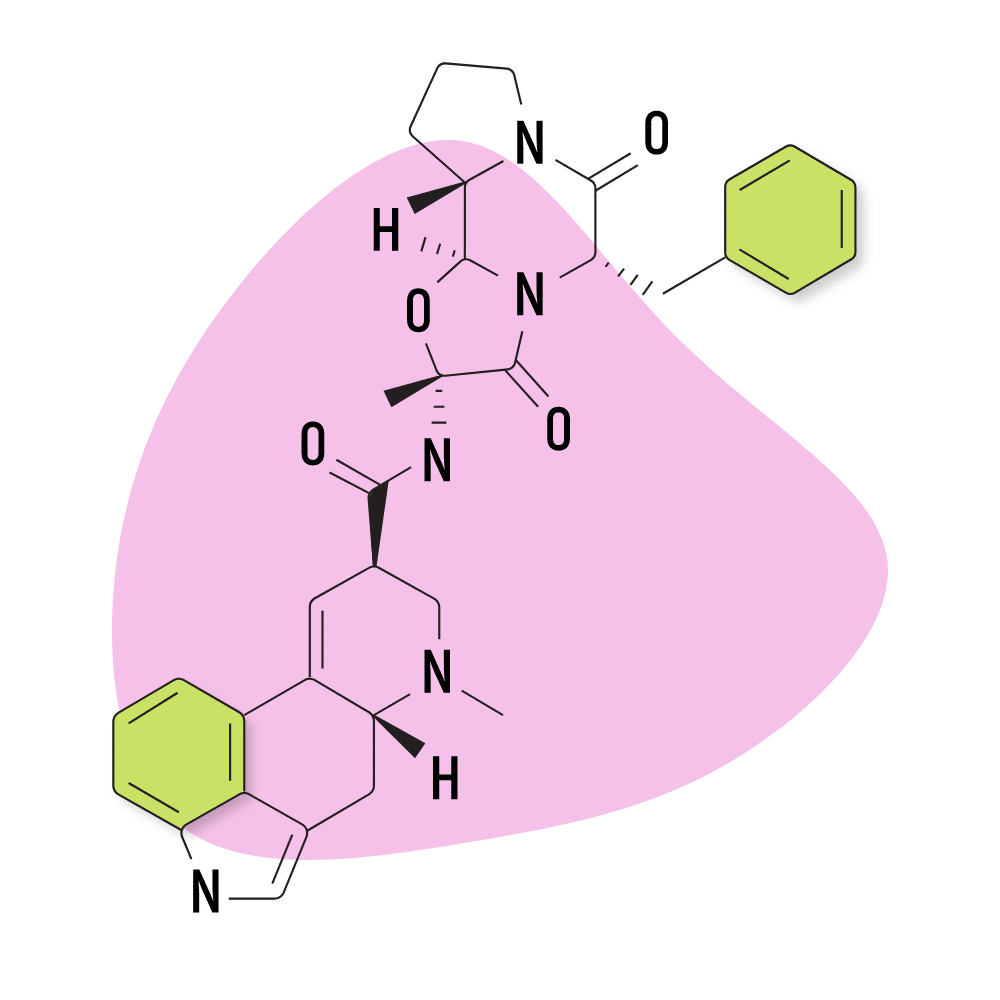
This compound is considered a controlled substance because it can be used as a precursor for making LSD.
4. Ergine
Ergine is the common name for LSA (lysergic acid amide), which was outlined earlier as one of the more commonly used lysergamides. This is probably the only naturally-occurring lysergamide used for its psychedelic effects. Most of the others produce terrible side effects in high doses.
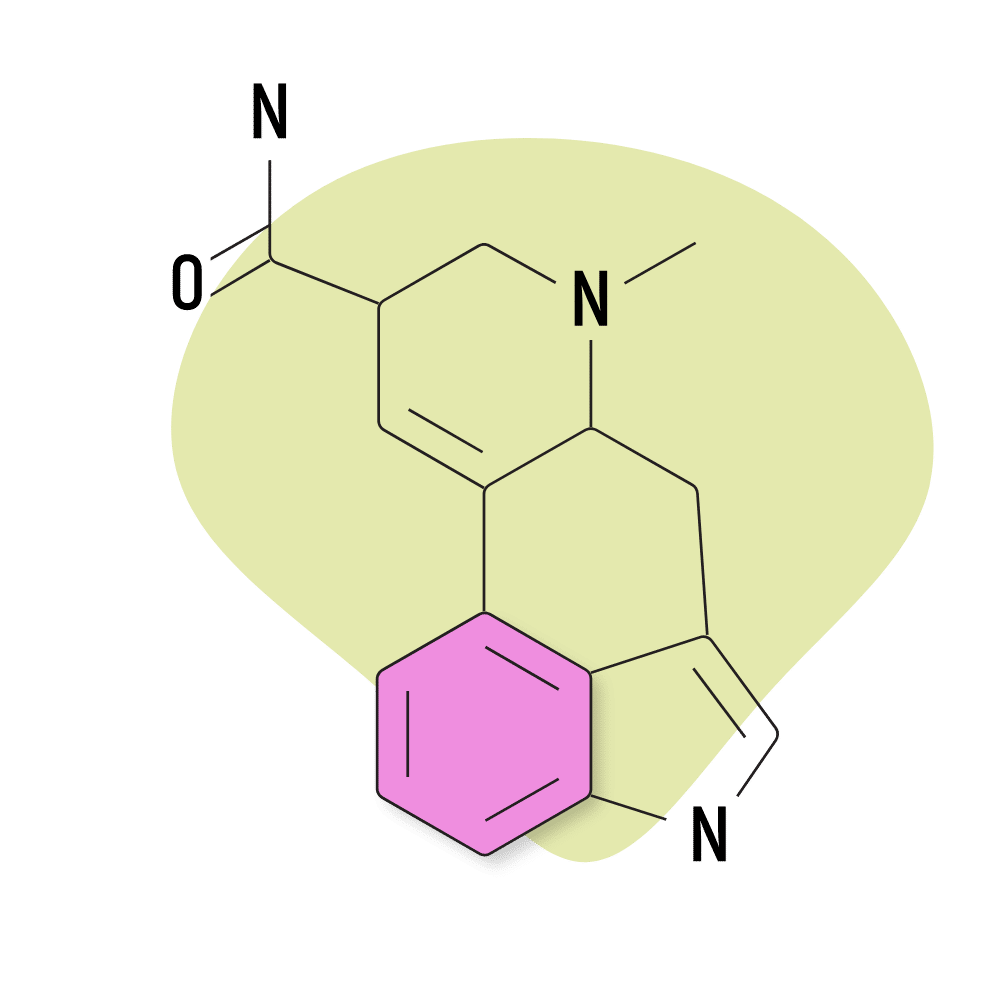
Ergine produces side effects, too — most commonly nausea, stomach cramps, dizziness, and sedation. However, unlike other members of the ergoline alkaloid class, ergine doesn’t appear to produce any lasting changes, nor does it have a strong effect on the vascular system.
Most people take ergine in the form of morning glory or Hawaiian baby woodrose seed tinctures or extracts.
Are Lysergamides Safe?
Most lysergamides closely related to LSD are considered “likely very safe,” — but there isn’t enough research available to confirm this. Only a handful of LSD derivatives have been tested for safety, but so far, none appear to pose any increased risk.
Most of the naturally-occurring ergot alkaloids from Claviceps spp. are dangerous and should not be used for psychedelic purposes.
The only lysergamide with any significant research behind it in terms of safety is LSD — and the results have been very impressive.
Over the past 60 years of research and use of LSD among the public, there’s no evidence to suggest it poses any significant threat to one’s physical health when used responsibly and within the standard dosage range of 50–400 micrograms.
Dr. David Nichols published a paper asking the question “Is LSD Dangerous” in 2018 [9]. In this paper, Nichols points out several reports of LSD-related deaths — all of which involved a monumental dose of LSD several hundred times higher than what can be deemed reasonable.
The best way to determine the toxicity of a compound is to find the LD50 — which refers to the dose needed to kill roughly 50% of the population it was administered to (usually rodents). So far, there’s no clear LD50 for LSD, even after dosing animals with over 1600 times the normal psychoactive dose (200–400 micrograms).
A study from 1977 proposed the lethal dose was around 100 mg [10], but many researchers believe the number to be much higher than this.
The biggest risk of using LSD and related lysergamides comes from accidental injury and adulteration with other, more dangerous compounds — namely NBOMe.
NBOMe substances (especially 25I-NBOMe) are one of the few compounds that offer comparable potency to lysergamides. Most other substances aren’t strong enough to provide a fatal hit in sub-milligram doses. The blotter paper lysergamides are doses that can only hold around 200 micrograms (0.2 mg) of the active ingredient. NBOMe substances have been fatal with as little as one tab but generally require two or more hits to result in death.
Are Lysergamides Legal?
No, lysergamides have been banned globally since the 1960s. Initially, just LSD was banned, but over the years other LSD derivatives have also been added to this list.
For several decades, clandestine drug manufacturers and regulators have been playing a game of cat and mouse. A new compound is created, such as ETH-LAD, which side-steps the regulations around LSD. Then a new law is imposed to ban ETH-LAD, so 1P-ETH-LAD is created instead.
Today, most countries have some form of analog act, which makes all psychoactive isomers of known psychedelics illegal by default. This means any new lysergamide created is already illegal, even if it isn’t specifically mentioned.
Some countries, such as Canada, don’t have analog laws, so certain lysergamides that aren’t on the list of controlled substances may still be legal. For example, many experts believe 1P-LSD, 1B-LSD, and 1V-LSD are all legal in Canada.
What’s The Strongest Lysergamide Psychedelic?
Anecdotal reports suggest ETH-LAD is the strongest member of the lysergamide class, but not by much. The threshold dose of this compound is somewhere around 20 micrograms, compared to 25 for LSZ and 25–30 for LSD. Most people who take a tab of ETH-LAD won’t be able to tell the difference from a tab of LSD.
It’s difficult to assess the potency of these compounds through anecdotes because it’s impossible to know the exact dosage used. A blotter tab can hold anywhere from 25–200 micrograms of active ingredient depending on how it was made and how long it’s been in storage.
One way to assess the potency of psychoactive compounds is to compare the binding capacity on target receptors like the 5HT2A receptor. However, this doesn’t provide the whole picture either. Just because something binds more strongly to a receptor doesn’t mean the effects it produces are stronger.
All this aside, based on the information currently available, here’s an estimated ranking of lysergamides by potency (strongest to weakest):
Are There Any Natural Lysergamides?
Yes, LSA is a naturally-occurring lysergamide found in the ergot fungus, as well as morning glory and Hawaiian baby Woodrose seeds. The common name for this compound is ergine.
There are other psychoactive ergot alkaloids that are classified as lysergamides, but most of them are toxic when used in psychoactive doses.
The vast majority of lysergamides used for their psychoactive effect are either synthetic or semi-synthetic.
Summary: What Are Lysergamides?
The lysergamides are a large group of psychedelic compounds closely related to LSD.
These compounds interact with both the serotonin and dopamine receptors in the brain to produce the characteristic psychoactive effects.
Lysergamides are exceptionally potent — most are active in doses well below a single milligram. They all produce similar effects but may provide subtle differences depending on how strongly they bind to various receptors in the brain. Some are more tactile and have a stronger body load; others are more cerebral and introspective, and some are lucid and sedating.
If you’re interested in trying lysergamides, it’s important to always test a sample of your substance before you take it, prioritize developing the proper set and setting, and avoid using psychedelics if you have any diagnosed psychiatric disorders or are taking lithium or other prescription medications.
Remember that most of the substances on this list are considered research chemicals — which means they lack crucial scientific research to prove them safe for human consumption.
If you have any comments or feedback that would improve the information in this resource, please email feedback@tripsitter.com.
Further Reading
- LSD: My Problem Child — Albert Hofmann
- Return of the lysergamides. Part I: Analytical and behavioural characterization of 1-propionyl-d-lysergic acid diethylamide (1P-LSD) [1].
- Return of the lysergamides. Part II: Analytical and behavioural characterization of N 6 -allyl-6-norlysergic acid diethylamide (AL-LAD) and (2’ S ,4’ S )-lysergic acid 2,4-dimethylazetidide (LSZ) [2].
- Return of the lysergamides. Part III: Analytical characterization of N6 -ethyl-6-norlysergic acid diethylamide (ETH-LAD) and 1-propionyl ETH-LAD (1P-ETH-LAD) [3].
- Return of the lysergamides. Part IV: Analytical and pharmacological characterization of lysergic acid morpholide (LSM-775) [4].
- Return of the lysergamides. Part V: Analytical and behavioural characterization of 1-butanoyl-d-lysergic acid diethylamide (1B‐LSD) [5].
- Return of the lysergamides. Part VI: Analytical and behavioural characterization of 1-cyclopropanoyl-d-lysergic acid diethylamide (1CP-LSD) [6].
References
- Brandt, S. D., Kavanagh, P. V., Westphal, F., Stratford, A., Elliott, S. P., Hoang, K., … & Halberstadt, A. L. (2016). Return of the lysergamides. Part I: Analytical and behavioural characterization of 1‐propionyl‐d‐lysergic acid diethylamide (1P‐LSD). Drug testing and analysis, 8(9), 891-902.
- Brandt, S. D., Kavanagh, P. V., Westphal, F., Elliott, S. P., Wallach, J., Colestock, T., … & Halberstadt, A. L. (2017). Return of the lysergamides. Part II: analytical and behavioural characterization of N6-allyl-6-norlysergic acid diethylamide (AL-LAD) and (2’S, 4’S)-lysergic acid 2, 4-dimethylazetidide (LSZ). Drug testing and analysis, 9(1), 38-50.
- Brandt, S. D., Kavanagh, P. V., Westphal, F., Elliott, S. P., Wallach, J., Stratford, A., … & Halberstadt, A. L. (2017). Return of the lysergamides. Part III: Analytical characterization of N6-ethyl-6-norlysergic acid diethylamide (ETH-LAD) and 1-propionyl ETH-LAD (1P–ETH-LAD). Drug testing and analysis, 9(10), 1641-1649.
- Brandt, S. D., Kavanagh, P. V., Twamley, B., Westphal, F., Elliott, S. P., Wallach, J., … & Halberstadt, A. L. (2018). Return of the lysergamides. Part IV: analytical and pharmacological characterization of lysergic acid morpholide (LSM-775). Drug testing and analysis, 10(2), 310-322.
- Brandt, S. D., Kavanagh, P. V., Westphal, F., Stratford, A., Elliott, S. P., Dowling, G., … & Halberstadt, A. L. (2019). Return of the lysergamides. Part V: Analytical and behavioural characterization of 1-butanoyl-d-lysergic acid diethylamide (1B‐LSD). Drug testing and analysis, 11(8), 1122-1133.
- Brandt, S. D., Kavanagh, P. V., Westphal, F., Stratford, A., Odland, A. U., Klein, A. K., … & Halberstadt, A. L. (2020). Return of the lysergamides. Part VI: Analytical and behavioural characterization of 1-cyclopropanoyl-d-lysergic acid diethylamide (1CP‐LSD). Drug testing and analysis, 12(6), 812-826.
- Juszczak, G. R., & Swiergiel, A. H. (2013). Recreational use of D-lysergamide from the seeds of Argyreia nervosa, Ipomoea tricolor, Ipomoea violacea, and Ipomoea purpurea in Poland. Journal of psychoactive drugs, 45(1), 79-93.
- Mehta, M. A., & Tricklebank, M. D. (2019). Serotonin and the psychedelics. In The Serotonin System (pp. 193-202). Academic Press.
- Smith, S., & Timmis, G. M. (1932). 98. The alkaloids of ergot. Part III. Ergine, a new base obtained by the degradation of ergotoxine and ergotinine. Journal of the Chemical Society (Resumed), 763-766.
- Nichols, D. E., & Grob, C. S. (2018). Is LSD toxic?. Forensic science international, 284, 141-145.
- Griggs, E. A., & Ward, M. (1977). LSD toxicity: a suspected cause of death. The Journal of the Kentucky Medical Association, 75(4), 172-173.