Tofisopam: Uses & Characteristics Of This Atypical Benzodiazepine
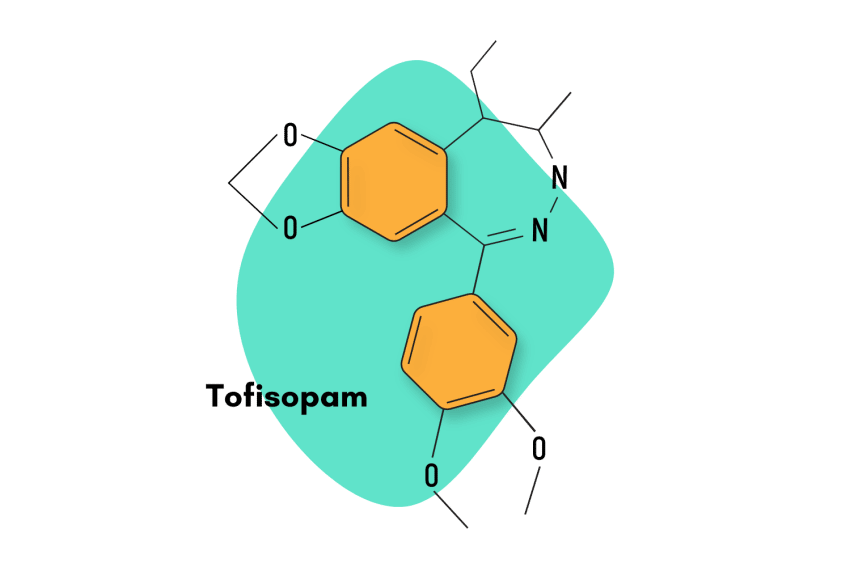
Tofisopam, sold under the brand names Emandaxin, Grandaxin, and Sériel, is a 2,3-benzodiazepine compound used primarily to boost the effects of other benzodiazepine sedatives.
The 2,3-benzodiazepine group is unique in that they don’t work through the usual GABAergic mechanisms. This family is considered “atypical” in that they target a totally different set of receptors (AMPA).
Tofisopam is indicated for the short-term management of anxiety and the relief of alcohol withdrawal syndrome. It’s being explored as a potential treatment for neurodegenerative disorders, including Alzheimer’s disease, Huntington’s disease, and amyotrophic lateral sclerosis (ALS).
Tofisopam is sold in some countries across Europe but is not an approved medication in the United States. A closely-related compound is currently being developed for clinical use by Vela Pharmaceuticals in New Jersey, USA.
Tofisopam Specs:
| Status: | Approved Medication |
| Common Dosage: | 50–100 mg (1-3 times daily) |
| PubChem ID: | 5502 |
| CAS# | 22345-47-7 |
IUPAC Name:
1-(3,4-dimethoxyphenyl)-5-ethyl-7,8-dimethoxy-4-methyl-5H-2,3-benzodiazepine
Other Names: Emandaxin, Grandaxin, and Sériel
Metabolism:
The metabolism of tofisopam is hepatic, and the main major route of biotransformation is mono-, di-, tri-, and tetra-o-demethylation in the various degree and positions of aromatic rings [2].
Tofisopam is primarily excreted in the urine (60-80%) in the form of conjugates with glucuronic acid and, to a lesser extent (about 30%), in feces.
The half-life is six to eight hours.
Tofisopam does not accumulate in the body, and its metabolites were not found to be pharmacologically active.
Duration of Effects:
Tofisopam reaches peak plasma levels in two hours and has an elimination half-life of six to eight; tofisopam is likely a short to intermediate-acting compound.
How Does Tofisopam Work?
Instead of offering GABA-A receptor potentiation like most other benzodiazepines, tofisopam binds to a receptor called the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor [1]. Tofisopam blocks the effects of this receptor to exert its effects.
AMPA receptor mediation has shown promise in the treatment of neurodegenerative disorders such as Parkinson’s, Alzheimer’s, Huntington’s, and amyotrophic lateral sclerosis (ALS).
Certain 2,3-benzos, including tofisopam, also display antipsychotic activity and may become effective treatments for conditions like schizophrenia [3]. Due to these developments, there is now significant scientific interest in understanding the molecular mechanisms which drive the functioning of the AMPA receptors.
But there’s still a lot about this interaction and the 2,3-benzodiazepine class, in general, we don’t yet understand.
Through some early research, we know some exclusive 2,3-benzodiazeoine binding occurs within the brain area known as the striatum [4]. The distribution of this proposed binding site overlaps largely with the distribution of the phosphodiesterase 10 isoenzyme (PDE-10), which is also a target for other antipsychotic agents [5].
These findings suggest that tofisopam’s mechanism of action comes not only as a result of AMPA receptor interaction but also through its inhibition of different PDE isoenzymes and subsequent effects [3].
It’s important to understand that even though tofisopam shares a chemical backbone with “classical” 1,4-benzodiazepines and also displays some of the same anxiolytic (anti-anxiety) properties, it does not interact with the benzodiazepine binding site located on GABA-A receptor [3].
Tofisopam does not display the sedative, hypnotic or muscle-relaxant effects usually associated with benzodiazepine compounds.
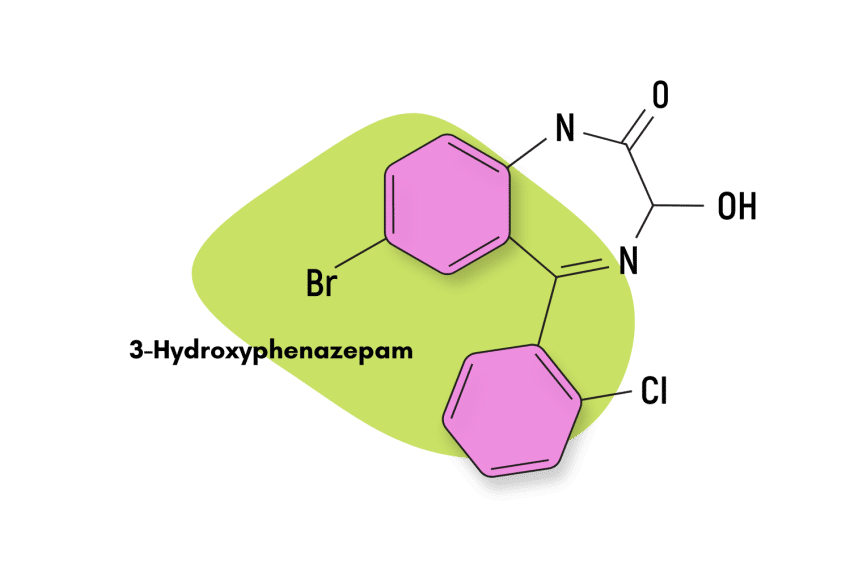
Is Tofisopam Safe? Risks & Side-Effects
Benzodiazepines that use the standard GABA-mediated pathway produce their effects through a generalized depressive effect on the central nervous system (CNS). This common mechanism is the reason why most benzodiazepines share the same contraindications, side effects, and properties, with only slight variations between them.
The general similarities exhibited by traditional benzodiazepines entail a baseline risk level that is common to all of them. This is why virtually all benzos are classified as Schedule IV compounds in the Controlled Substances Act.
However, tofisopam is a little bit different.
In general terms, the lack of a depressive effect on the CNS gives tofisopam a better risk profile than most other benzodiazepines. The risk of respiratory depression, disinhibition, amnesia, and sedation are minimal.
The lack of GABA mediation also makes tofisopam far less likely to lead to physical dependence among users. However, tofisopam has been found on designer drug markets, which suggests that it does have at least some potential for recreational use.
Tofisopam is also known to be an inhibitor of the CYP3A4 enzyme, which can be quite troublesome. The CYP3A4 enzyme is responsible for the metabolic clearance of a vast number of compounds. When its performance is inhibited by extraneous compounds, it can lead to an accumulation of tofisopam or other 3A4-mediated drugs in the body [6].
In general, researchers are confident this medication presents fewer overall risks than traditional benzodiazepine use.
Side Effects of Tofisopam
The side effects of tofisopam may include [3]:
- Agitation
- Confusion
- Constipation
- Convulsions (in patients with epilepsy)
- Decreased appetite
- Dry Mouth
- Headache
- Increased flatus
- Increased irritability
- Insomnia
- Muscle pain
- Muscle tension
- Nausea
- Obstructive jaundice
Benzodiazepine Withdrawal & Dependence
Due to its unique mechanisms of action, tofisopam displays a reduced dependence liability when compared to typical benzodiazepines. However, there are practically no studies that directly attempt to assess the likelihood of tofisopam dependence on users. In any case, doctors seem to think it is not a suitable compound for long-term use, which implies that, over a long period of time, some form of dependence is possible.
Harm Reduction: Tofisopam
Benzodiazepine Harm Reduction Tips:
- 🥣 Don’t mix — Mixing benzodiazepines with other depressants (alcohol, GHB, phenibut, barbiturates, opiates) can be fatal.
- ⏳ Take frequent breaks or plan for a short treatment span — Benzodiazepines can form dependence quickly, so it’s important to stop using the drug periodically.
- 🥄 Always stick to the proper dose — The dosage of benzos can vary substantially. Some drugs require 20 or 30 mg; others can be fatal in doses as low as 3 mg.
- 💊 Be aware of contraindications — Benzodiazepines are significantly more dangerous in older people or those with certain medical conditions.
- 🧪 Test your drugs — If ordering benzos from unregistered vendors (online or street vendors), order a benzo test kit to ensure your pills contain what you think they do.
- 💉 Never snort or inject benzos — Not only does this provide no advantage, but it’s also extremely dangerous. Benzos should be taken orally.
- 🌧 Recognize the signs of addiction — Early warning signs are feeling like you’re not “yourself” without the drug or hiding your habits from loved ones.
- ⚖️ Understand the laws where you live — In most parts of the world, benzodiazepines are only considered legal if given a prescription by a medical doctor.
- 📞 Know where to go if you need help — Help is available for benzodiazepine addiction; you just have to ask for it. Look up “addiction hotline” for more information where you live. (USA: 1-800-662-4357; Canada: 1-866-585-0445; UK: 0300-999-1212).
Tofisopam Drug Interactions
The simultaneous use of tacrolimus, sirolimus, cyclosporine, and tofisopam is contraindicated. Medicines, which are metabolized by CYP3A4, can increase their concentration in blood plasma in case of simultaneous admission with tofisopam.
The use of tofisopam with medicines suppressing the central nervous system (analgesics, general anesthesia medicines, antidepressants, H1-antihistamines, sedatives, hypnotics, and antipsychotics) may interact with tofisopam by enhancing their effects. This could lead to excessive sedation, disinhibition, and respiratory depression.
Liver enzyme inductors (barbiturates, alcohol, nicotine, antiepileptic medicines) may increase the metabolism of tofisopam — leading to faster elimination and reduced therapeutic effect.
Some antifungal medicines (ketoconazole, itraconazole) can slow the hepatic metabolism of tofisopam, which leads to an increase in its concentration in the blood plasma.
Some antihypertensive medicines (clonidine, calcium channel antagonists) may enhance the effects of tofisopam.
Tofisopam Contraindications
Contraindications refer to specific conditions in which the drug carries excessively high risk. Doctors use contraindications to minimize the level of risk when prescribing a medication.
This is essentially the list of situations in which users should avoid using tofisopam at all costs.
Contraindications of tofisopam include:
- Acute respiratory failure
- Glaucoma
- Decompensated respiratory failure
- Epilepsy
- The first trimester of pregnancy and breastfeeding
- Hypersensitivity to other benzodiazepines
- Intolerance to galactose
- Organic brain lesions (such as atherosclerosis)
- Simultaneous use of tacrolimus, sirolimus, or cyclosporin
- Sleep apnea
Tofisopam Dosage
The standard tofisopam dosage ranges include:
- Adults: 50 – 100 mg (1-2 tablets) taken 1-3 times a day. The maximum daily dose is 300 mg.
- Elderly and impaired patients: the daily dose should be reduced by approximately half.
With alcohol withdrawal syndrome, as well as for the prevention and treatment of delirium, a short course of therapy is indicated (from several days to 3-6 weeks).
Similar Benzodiazepines
Tofisopam is unlike most benzodiazepine drugs. It’s classified as a 2,3-benzodiazepine. Other closely-related members of this group include:
Girisopam
Girisopam, also known as GYKI-51189 and EGIS-5810, is regarded by researchers as being closely related to tofisopam and sometapine.
Like tofisopam, girisopam has anxiolytic properties but achieves these effects through pathways other than GABA-A mediation. They also share a lack of sedative and muscle-relaxant qualities.
Nerisopam
There is not a lot of research behind nerosipam, and the few studies which have been performed concluded its mechanism of action is not the same as tofisopam. However, tofisopam and nerisopam produce comparable effects on the user: both compounds are anxiolytics that lack the sedative and muscle-relaxant qualities of traditional benzodiazepines.
Zometapine
Zometapine is not a 2,3- benzodiazepine but rather a pyrazolodiazepine with some thienodiazepine characteristics. Despite holding some structural characteristics to certain 2,3-benzos, it’s similar to tofisopam in the sense they’re both “atypical antidepressants” with little psychotropic potential.
Tofisopam FAQs
What is the structural difference of 2,3- benzodiazepines when compared with traditional benzos?
2,3- benzodiazepines have a 2,3-benzodiazepine ring rather than the classical nitrogen atom positioning at 1,4.
What is the absorption of tofisopam like?
The medicine is quickly and almost completely absorbed from the gastrointestinal tract, with peak plasma concentrations achieved within 2 hours of a single dose, and has a half-life of 6 hours [7].
Where was tofisopam originally developed?
Tofisopam was originally developed in Hungary in the 1970s for use as a prescription benzodiazepine and subsequently licensed in some European countries for the treatment of neurotic and somatic disorders (including anxiety, vegetative disorders, apathy, fatigue, and low mood).
What is tofisopam’s psychotropic profile?
In a study that directly compared the psychotropic profiles of tofisopam and diazepam, it was shown how these two compounds are immensely distinct. Diazepam showed a clear profile of action, producing EEG changes, pronounced sedation, and psychological impairment, while produced no changes on the EEG or psychological tests, and only a very mild stimulant effect was apparent on the ratings [8].
References
- Qneibi, M., Jaradat, N., Hawash, M., Olgac, A., & Emwas, N. (2020). Ortho versus meta chlorophenyl-2, 3-benzodiazepine analogs: synthesis, molecular modeling, and biological activity as AMPAR antagonists. ACS omega, 5(7), 3588-3595.
- Klebovich, I., & Abermann, M. (1993). Pharmacokinetics and metabolism of tofizopam (Grandaxin). Acta Pharmaceutica Hungarica, 63(2), 83-90.
- Rundfeldt, C., Socała, K., & Wlaź, P. (2010). The atypical anxiolytic drug, tofisopam, selectively blocks phosphodiesterase isoenzymes and is active in the mouse model of negative symptoms of psychosis. Journal of neural transmission, 117(11), 1319-1325.
- Horváth, E. J., Horváth, K., Hámori, T., Fekete, M. I., Sólyom, S., & Palkovits, M. (2000). Anxiolytic 2, 3-benzodiazepines, their specific binding to the basal ganglia. Progress in neurobiology, 60(4), 309-342.
- Siuciak, J. A., Chapin, D. S., Harms, J. F., Lebel, L. A., McCarthy, S. A., Chambers, L., … & Schmidt, C. J. (2006). Inhibition of the striatum-enriched phosphodiesterase PDE10A: a novel approach to the treatment of psychosis. Neuropharmacology, 51(2), 386-396.
- Tóth, M., Bajnógel, J., Egyed, A., Drabant, S., Tömlo, J., & Klebovich, I. (2005). Effect of tofisopam on CYP3A4 enzyme activity on human recombinant 3A4 supersome. Acta Pharmaceutica Hungarica, 75(4), 195-198.
- Wolfe, C. (2022). Novel benzodiazepines. Novel Psychoactive Substances, 475-494.
- Bond, A., & Lader, M. (1982). A comparison of the psychotropic profiles of tofisopam and diazepam. European Journal of Clinical Pharmacology, 22(2), 137-142.