Ketamine Drug Class: Dissociative Anesthetic, Arylcyclohexylamine, Antidepressant, & More
Chemically, ketamine is classified as an arylcyclohexylamine. Pharmacologically, it’s a dissociative anesthetic. But there are other classifications for this enigmatic compound as well…
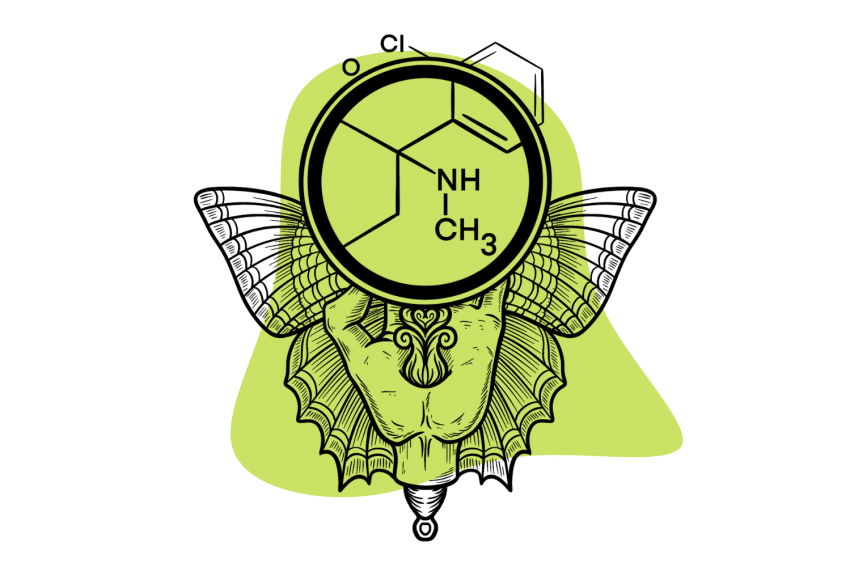
Ketamine is hard to fit into one box. Its impressive range of target receptors in the human body means it can do many different things.
In order to answer this question, we’ve broken the answer down into two parts:
Let’s dive straight in.
What is Ketamine?
Ketamine ((RS)-2-(2-chlorophenyl)-2-(methylamino)cyclohexanone) is a powerful psychoactive drug with the unique ability to decouple one’s conscious experience from the body. The effects of ketamine are bizarre and profoundly psychedelic.
Users often experience an altered perception of time, a sense of depersonalization (a feeling of detachment or estrangement from oneself), emotional numbness, and dramatic hallucinations (teleportation to other places or alternate universes).
Ketamine was invented in 1962 and quickly found itself in clinical practice as a replacement for PCP (phencyclidine) — which was being used at the time as an anesthetic during surgery. Ketamine was preferred for having fewer side effects than PCP, which occasionally caused patients to wake up in a state of psychotic delirium lasting several hours.
Today, ketamine is still used in anesthesia — particularly for oral surgeries. It’s also used as a treatment for depression, post-traumatic stress disorder, traumatic brain injuries, and migraine headaches. Some people take microdoses of ketamine as a nootropic to improve learning and memory (not recommended).
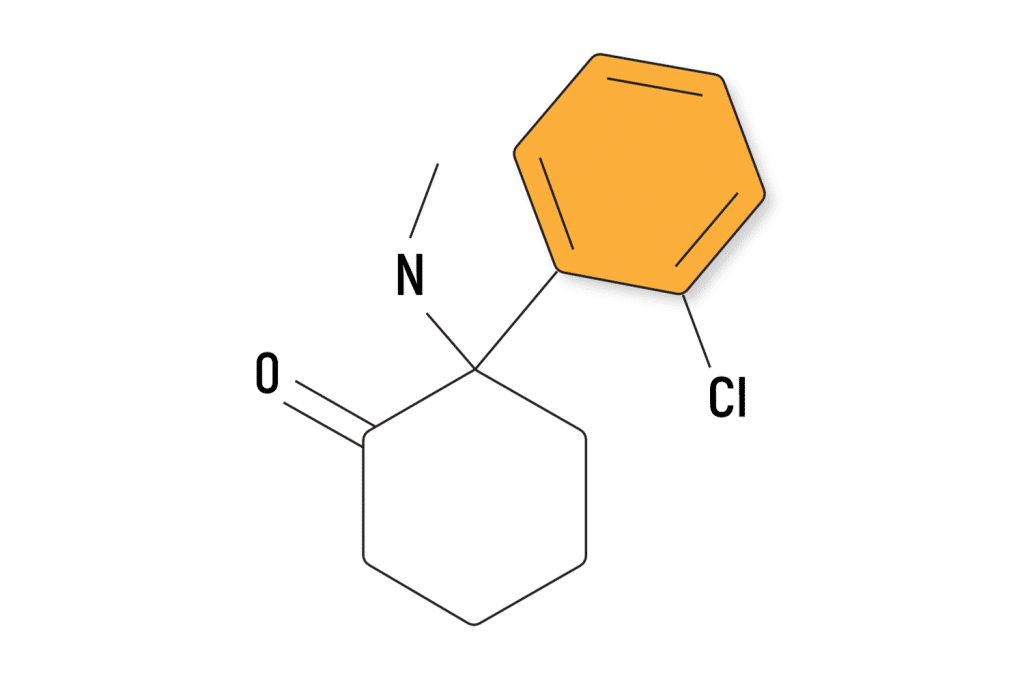
Ketamine Chemical Classification
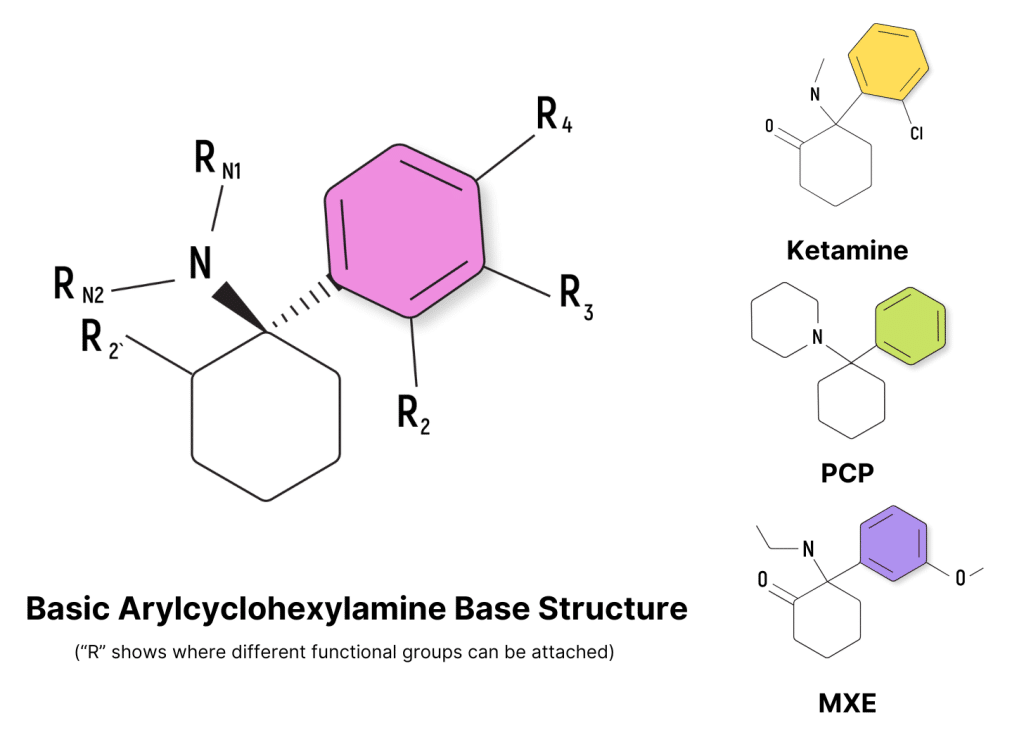
Ketamine is classified as an arylcyclohexylamine — which is a group of drugs characterized by the presence of a cyclohexane ring bound to an aromatic ring and containing an amine group.
There are hundreds of known compounds that fit this description — but none are as well established as ketamine. The vast majority of these drugs are classified as “research chemicals” or “designer drugs,” — which means we know very little about what they do, how they work, or what risks they carry.
Other well-known members of the arylcyclohexylamine family include PCP (phencyclidine), MXE (methoxetamine), DCK (deschloroketamine), and O-PCE.
Ketamine Pharmacological Classification
Classifying ketamine according to its effects is more complicated — ketamine fits into several different classes.
Generally speaking, ketamine is most often classified as a dissociative anesthetic.
The following list of ketamine classes is listed in order of relevance.
1. Dissociative
A dissociative drug refers to any compound that induces feelings of detachment from one’s self or the environment (called dissociation).
Ketamine owes its dissociative effect to its impact on the NMDA receptors — which are a type of glutamate receptor found throughout the brain involved in consciousness, memory, and other higher executive functioning.
Other common dissociatives include xenon gas, nitrous oxide gas, MK-801, dextromethorphan (DXM), salvinorin A (from Salvia divinorum), and most other arylcyclohexylamine drugs (including PCP, MXE, and DCK).
2. Anesthetic
Anesthetic drugs are substances used to induce a temporary state of unconsciousness, amnesia, or insensibility to pain for medical procedures — this state is called anesthesia.
Ketamine is considered a premier anesthetic because it’s very safe to use (unlike drugs such as halothane, which are known to damage liver and kidney tissue); it’s extremely reliable and is unlikely to leave users feeling nauseous or delirious after it wears off.
Ketamine kicks in quickly when injected and wears off quickly once the surgery is finished.
The powerful dissociative state induced by ketamine means users don’t feel or remember anything that happened during the surgery.
The only catch is that ketamine is not suitable for people with heart conditions and is too short-acting for longer surgical procedures.
3. Psychedelic
A psychedelic is considered any plant or substance that produces alterations in one’s visual, auditory, or tactile perception and induces altered states of consciousness.
While ketamine is distinct from conventional psychedelics, such as DMT (dimethyltryptamine), psilocybin (the active ingredient in magic mushrooms), LSD (lysergic acid diethylamide), or mescaline (the active compound in San Pedro and peyote cacti) — it does produce an experience that’s undeniably psychedelic in nature.
Many users experience profound visual hallucinations and sensations while under the effects of ketamine. Most of the time, these experiences are so bizarre it’s nearly impossible to explain or remember the details.
Examples of some descriptions of the ketamine experience I’ve heard from others include:
- “I felt like I was sitting beside myself.”
- “I was sucked into a vortex of colors I’ve never seen before.”
- “The world was being flushed down a giant cosmic drain.”
Related: Is Ketamine a Psychedelic?

4. Antidepressant
Antidepressant drugs come in many different forms. Some target serotonin (such as SSRIs like fluoxetine or sertraline), some focus on dopamine (such as bupropion), and others affect multiple targets at the same time.
Ketamine is unique because it targets the NMDA receptors — which makes it completely unique from every other antidepressant drug available today. Medical researchers still can’t agree on the exact role these receptors play in the pathophysiology of depression.
However, one thing remains clear — ketamine exerts a rapid (albeit short-lived) antidepressant effect. There have been hundreds of studies on this effect, with improvements ranging from 25% to 85% in the first 24 hours and 14% to 72% in the first 72 hours after treatment [1].
What’s even more impressive is that ketamine has shown a high incidence of antidepressant action in people suffering from treatment-resistant depression [2] — which is defined as patients who have had little to no improvement from at least two other accepted medical interventions.
The problem is that these effects require continual ketamine treatment every few weeks. One study reported that 8 of 9 patients who previously reported an impressive reduction in depression scores (a mean of 85%) relapsed into clinical depression within 19 days of concluding the treatment [3].
5. Nootropic
Nootropic derives from the root words “mind” and “bending” — so it refers to drugs that help the mind become more flexible. They’re used to improve one’s capacity for learning, memory, and cognitive functions.
Ketamine technically classifies as a nootropic because of its unique ability to promote neuroplasticity — which is the brain’s ability to form and reorganize synaptic connections, especially in response to learning or following injury.
Several studies have reported an increase in something called “spinogenesis” after exposure to ketamine [4]. Spinogenesis refers to the process of forming new dendritic spines on neurons. These spines are small, protruding structures found on neurons that play a key role in the formation of synaptic connectivity and transmission. Spinogenesis is a key aspect of neuroplasticity, the brain’s ability to reorganize itself by forming new neural connections.
The idea is that by increasing spinogenesis, ketamine helps the brain form new synaptic connections.
Most of the research on this effect focuses on the use of ketamine as a treatment for depression and traumatic brain injuries — but these same principles could be useful for healthy individuals as a nootropic, too.

6. Analgesic
Analgesics generally refer to substances that alleviate pain without causing loss of consciousness. Ketamine does cause loss of consciousness, but only in the higher dosage range. Analgesic effects have been reported at lower doses of ketamine [5] — though this is considered one of its minor functions.
Ketamine acts on pain through several key mechanisms — some of which are shared by classical painkillers; others are exclusive to ketamine.
The main analgesic action is believed to come from its interaction with the NMDA receptors — which is a highly unconventional target for pain meds. Ketamine’s impact on this receptor is believed to be a key contributor to the “wind up” phenomenon — where people experience increased sensitivity to pain. This could explain why low-dose ketamine is so useful for “pathological” pain states, such as fibromyalgia, neuropathic pain, migraine headaches, or complex regional pain syndrome (CRPS).
Ketamine has also been shown to act on the mu-opioid receptors [6], which act to regulate pain signals before they reach the brain. This is the same mechanism of action opiates like oxycodone or morphine — albeit significantly milder.
It’s important to note that ketamine can also cause certain types of pain, specifically bladder pain. This is a known side effect of frequent or high-dose ketamine consumption. Metabolites, such as norketamine, end up in the bladder, where they erode the protective lining.
Ketamine-induced bladder pain normally subsides quickly upon ceasing use of the drug.

Subscribe to Tripsitter: Newsletter & Podcast
Unlock Your Mind: Subscribe for Expert Insights on Psychedelics🍄🌵
References
- Aan Het Rot, M., Zarate Jr, C. A., Charney, D. S., & Mathew, S. J. (2012). Ketamine for depression: where do we go from here? Biological psychiatry, 72(7), 537-547. https://doi.org/10.1016/j.biopsych.2012.05.003
- Phillips, J. L., Norris, S., Talbot, J., Birmingham, M., Hatchard, T., Ortiz, A., … & Blier, P. (2019). Single, repeated, and maintenance ketamine infusions for treatment-resistant depression: a randomized controlled trial. American Journal of Psychiatry, 176(5), 401-409. https://doi.org/10.1176/appi.ajp.2018.18070834
- Aan Het Rot, M., Collins, K. A., Murrough, J. W., Perez, A. M., Reich, D. L., Charney, D. S., & Mathew, S. J. (2010). Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biological psychiatry, 67(2), 139-145. https://doi.org/10.1016/j.biopsych.2009.08.038
- Wu, H., Savalia, N. K., & Kwan, A. C. (2021). Ketamine for a boost of neural plasticity: how, but also when? Biological Psychiatry, 89(11), 1030-1032. https://doi.org/10.1016/j.biopsych.2021.03.014
- Visser, E., & Schug, S. A. (2006). The role of ketamine in pain management. Biomedicine & Pharmacotherapy, 60(7), 341-348. https://doi.org/10.1016/j.biopha.2006.06.021
- Levinstein, M. R., Carlton, M. L., Di Ianni, T., Ventriglia, E. N., Rizzo, A., Gomez, J. L., … & Michaelides, M. (2023). Mu opioid receptor activation mediates (S)-ketamine reinforcement in rats: implications for abuse liability. Biological Psychiatry, 93(12), 1118-1126. https://doi.org/10.1016/j.biopsych.2022.12.019
- Herrero, J. F., Laird, J. M., & Lopez-Garcia, J. A. (2000). Wind-up of spinal cord neurons and pain sensation: much ado about something? Progress in neurobiology, 61(2), 169-203. https://doi.org/10.1016/S0301-0082(99)00051-9