What Are Psychedelic Amphetamines? (List of 50+ Psychoactive Amphetamines)
There’s more to the amphetamine family than just MDMA. Learn about more than 50 prominent amphetamines with known psychoactive effects 💊
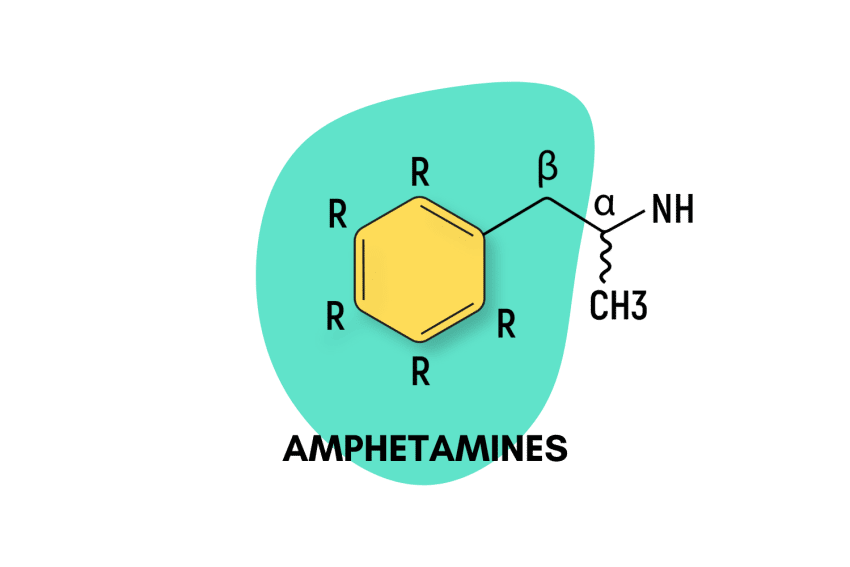
Psychedelic amphetamines make up the bulk of the “entactogen” class of drugs. They work by altering the chemistry of our brains to induce feelings of pleasure and motivation, enhance our ability to empathize with others, and increase energy levels.
The amphetamine family is incredibly diverse, but we can break them down into just 5 main categories:
- Basic amphetamines like Adderall and crystal meth
- Fluorinated amphetamines such as 2-FA and 4-FA
- MDXX amphetamines like MDMA and MDA
- Substituted cathinones like MDPV and α-PVP
- DOX compounds such as DOB and DOI
Here, we’ll examine each of the major amphetamine classes in detail, including their most prominent members. We’ll cover what the doses look like, their potential for harm, and what research (if any) is available for each substance.
What Are Amphetamines?
Amphetamines are a class of central nervous system stimulants. Some are used as a treatment for ADHD, obesity, Parkinson’s disease, or narcolepsy; others are used as recreational drugs.
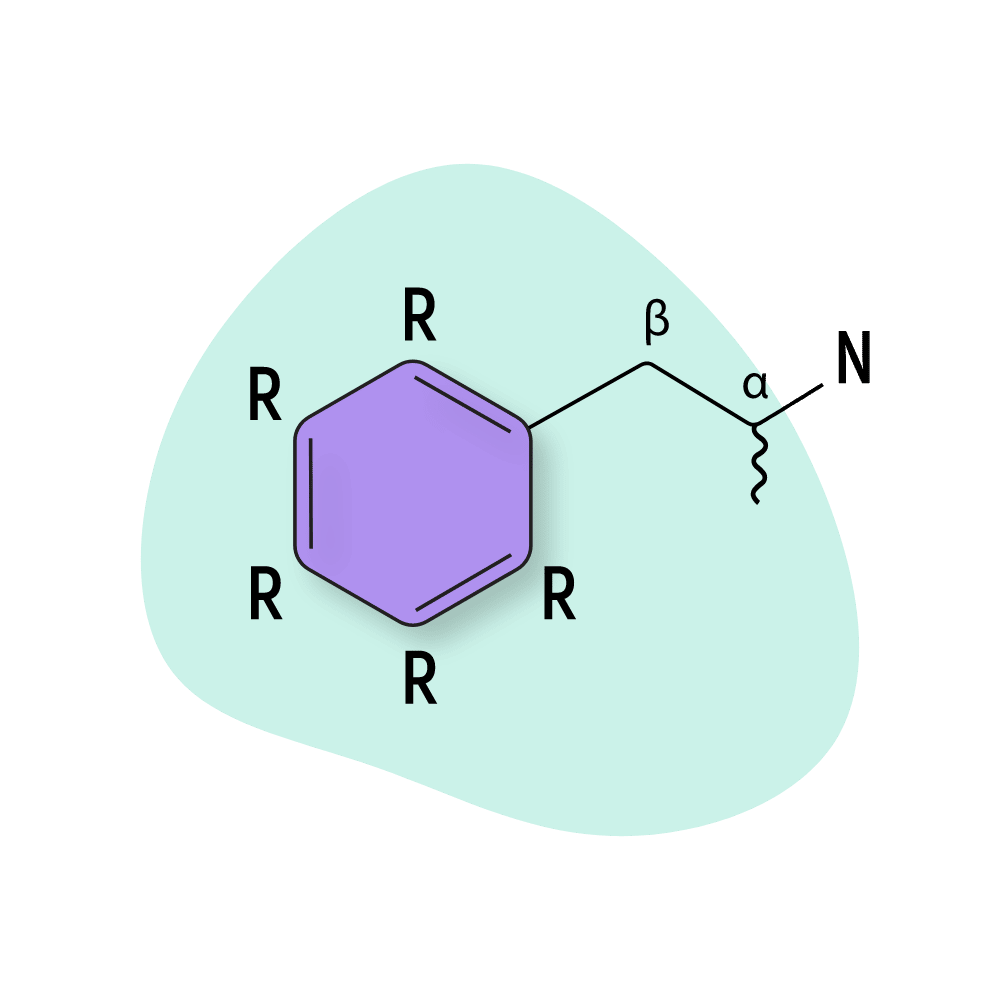
All amphetamines are derived from the basic amphetamine molecule. New amphetamine derivatives are made by swapping one or more of the hydrogen atoms attached to the central structure with other functional groups.
The effects of amphetamines vary from being stimulating like caffeine, to psychedelic like mescaline — and everything in between. Most amphetamines act as stimulants and euphorics (mood-enhancers).
List of Common Amphetamines
| Image | Name | Status | Duration of Effects | Estimated Threshold Dose (Oral) |
| 2-FA | RC 🧪 | 3–5 hours | 5 mg |
| 2-FEA | RC 🧪 | 1–2 hours | 10 mg |
| 2-FMA | RC 🧪 | 6–8 hours | 10 mg |
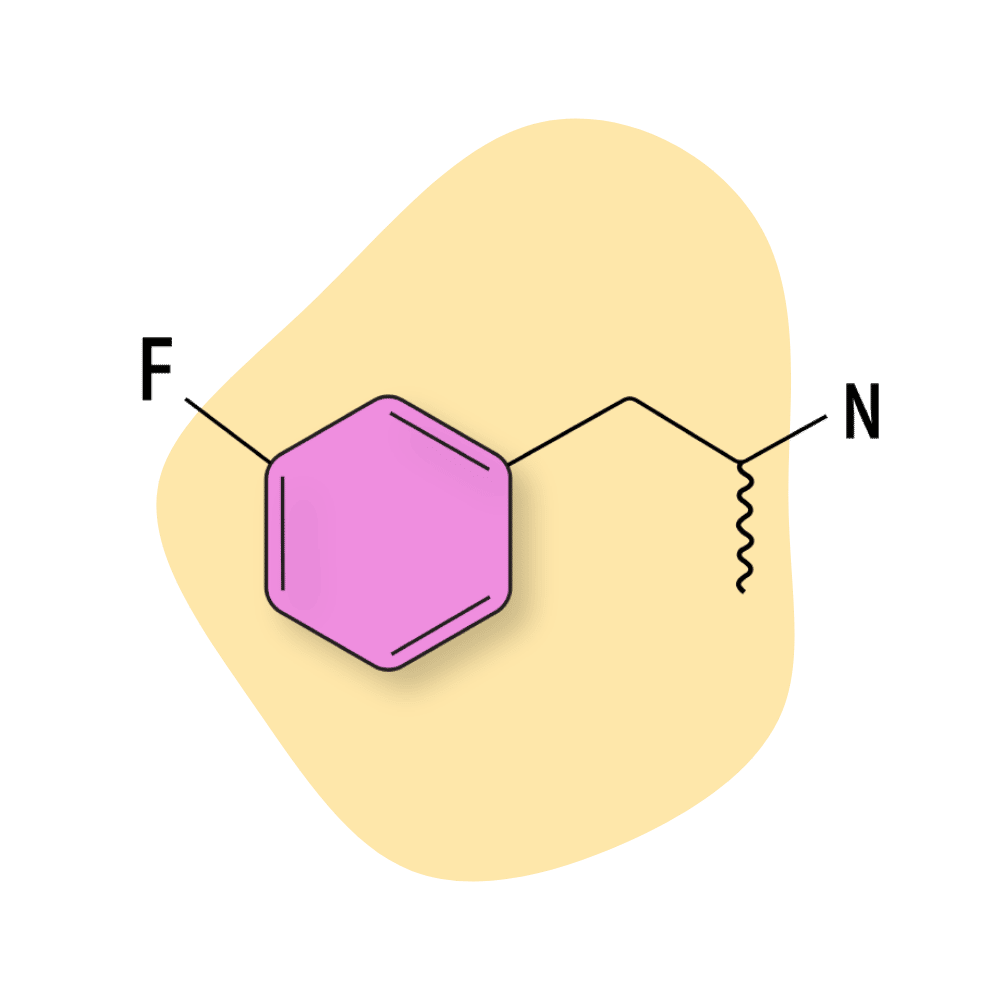
| 3-FA | RC 🧪 | 4–6 hours | 5 mg |
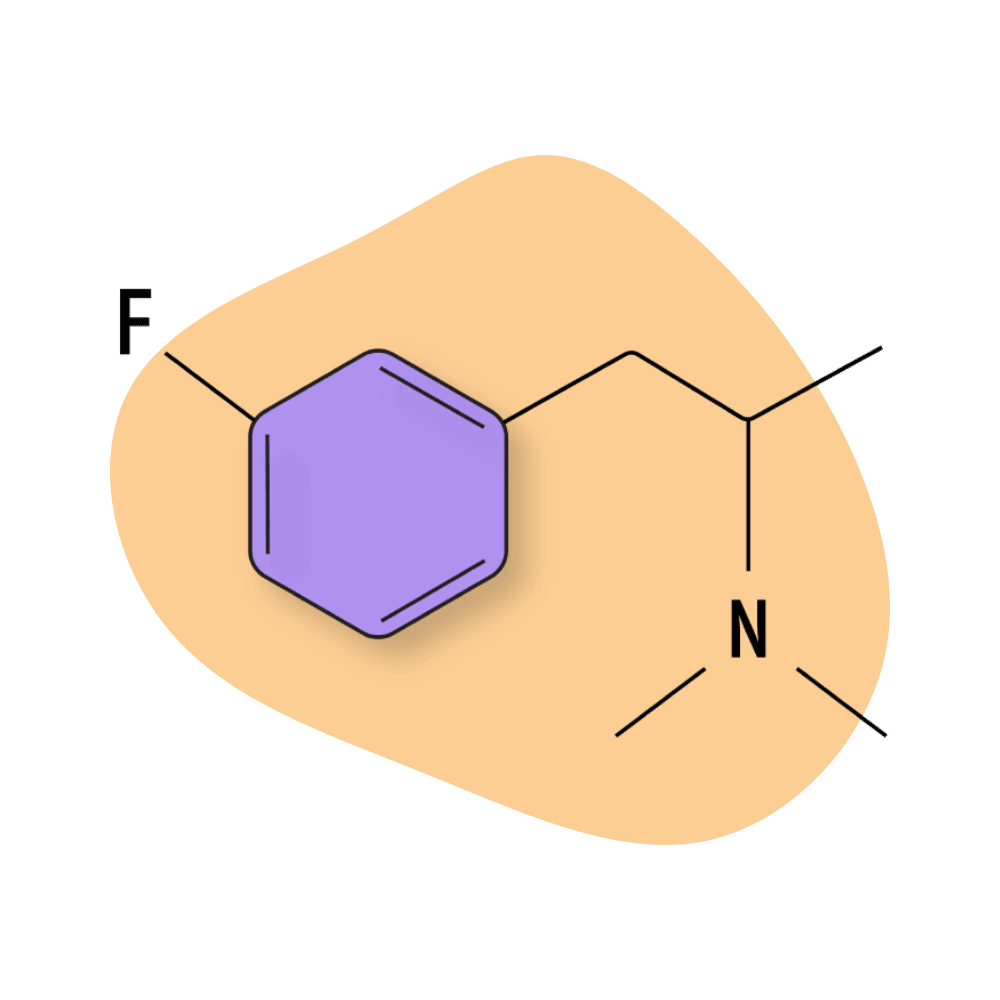
| 3-FMA | RC 🧪 | 4–8 hours | 5 mg |
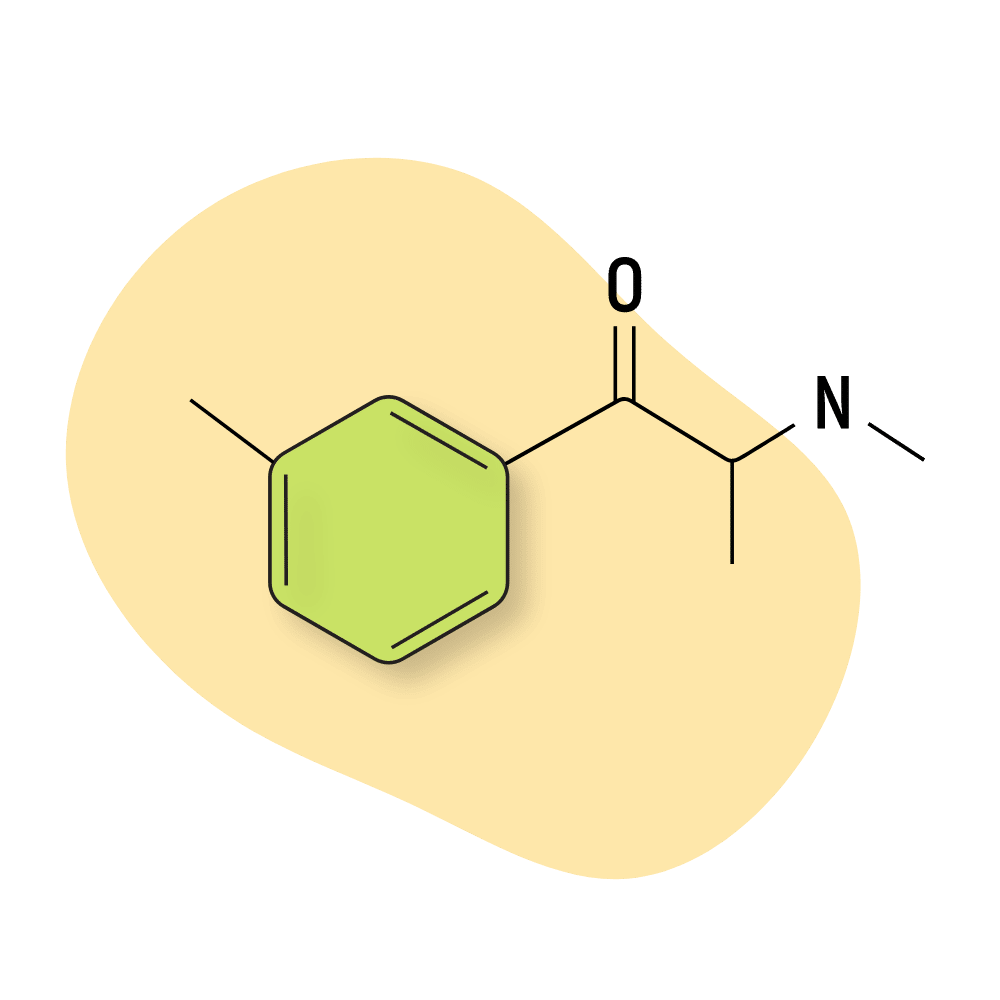
| 3-MMC | RC 🧪 | 4–7 hours | 20 mg |
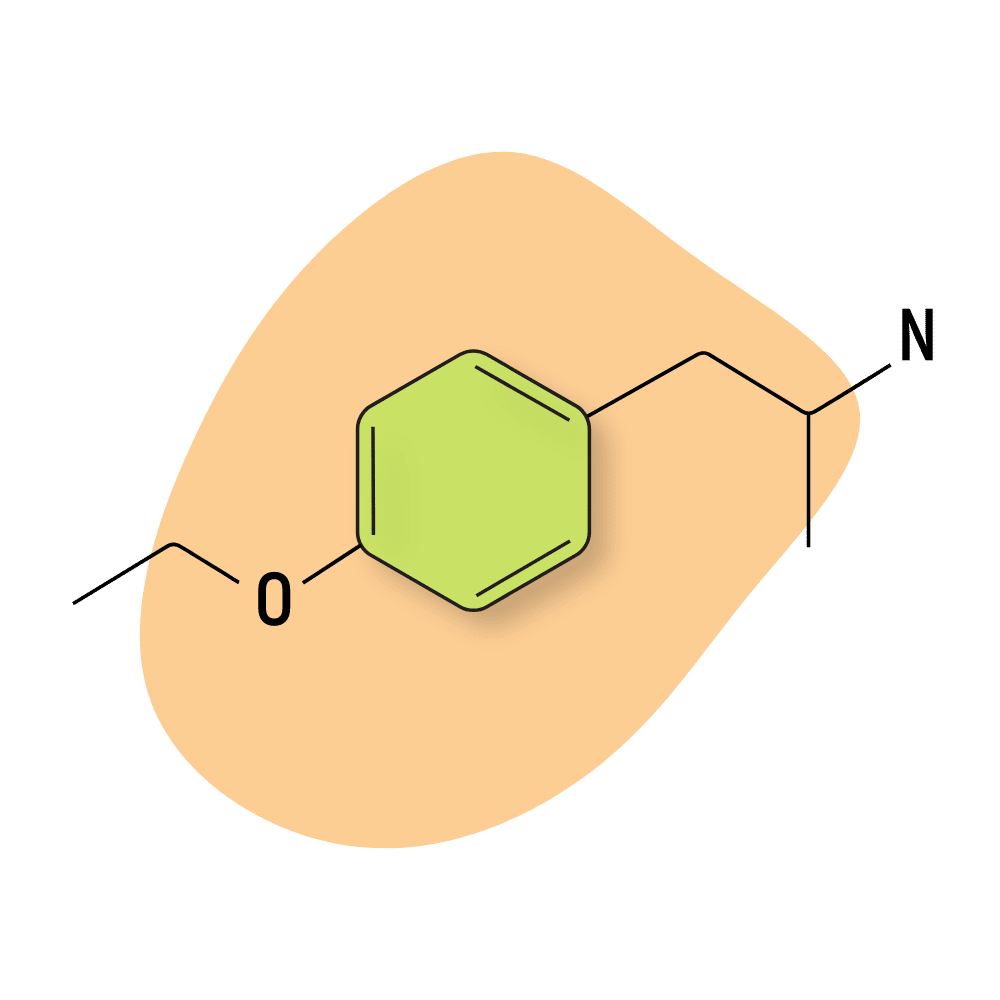
| 4-ETA | RC 🧪 | 10–14 hours | 10 mg |
| 4-FA | RC 🧪 | 5–8 hours | 20 mg |
| 4-FMA | RC 🧪 | 3–5 hours | 20 mg |
| Aleph-2 | RC 🧪 | 8–16 hours | 3 mg |
| Aleph-4 | RC 🧪 | 12–20 hours | 4 mg |
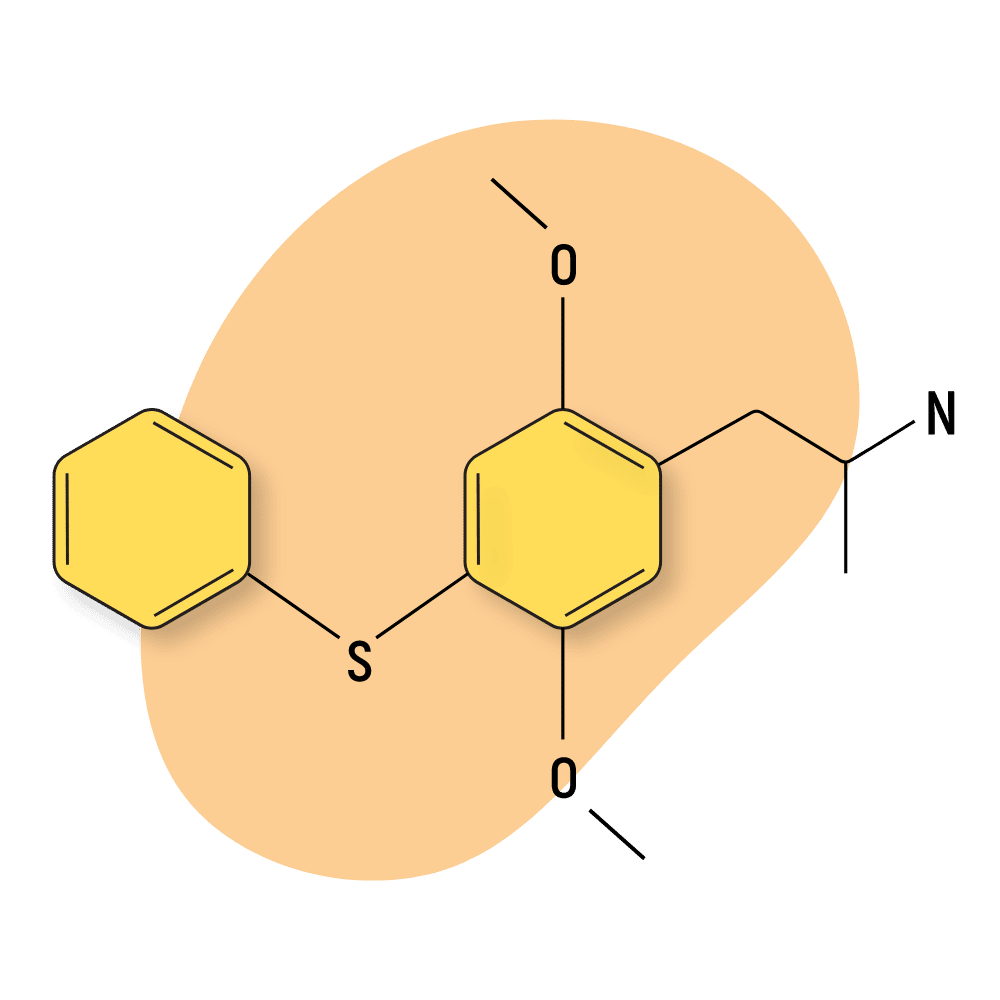
| Aleph-6 | RC 🧪 | Unknown (Long) | 20 mg |
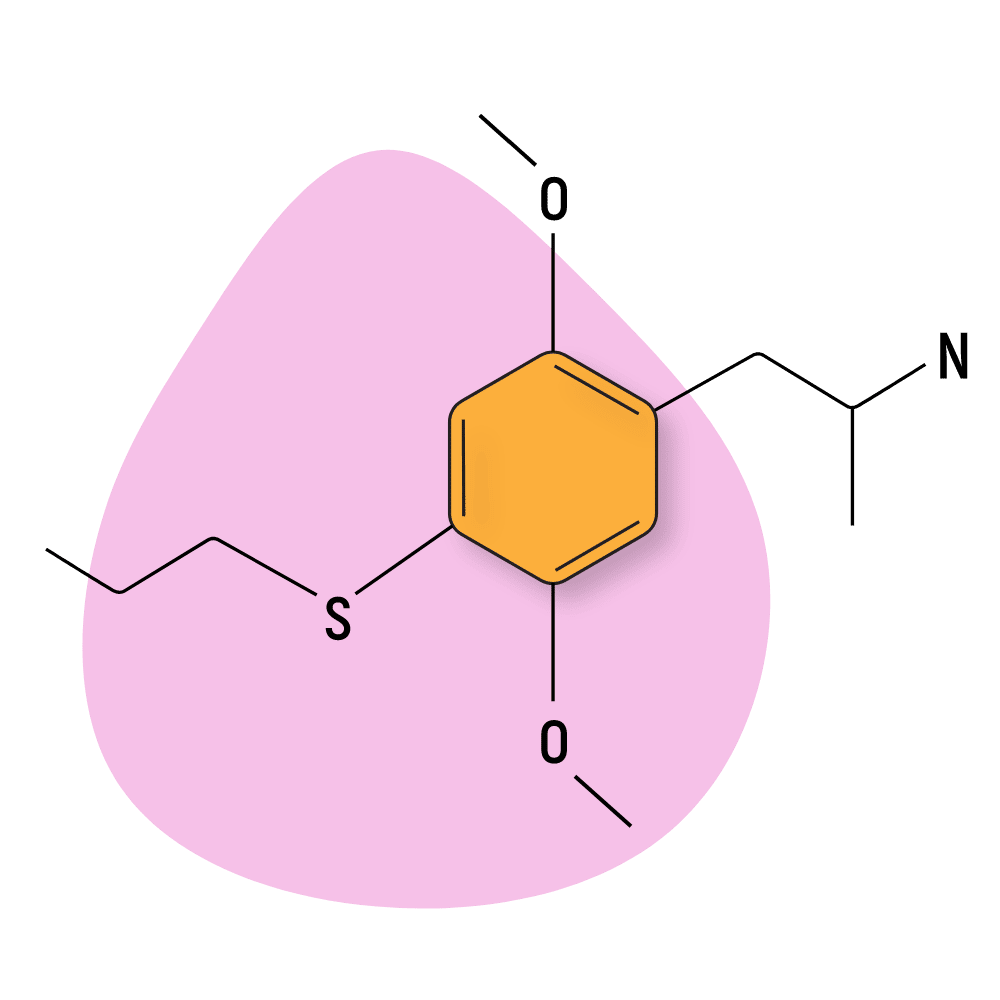
| Aleph-7 | RC 🧪 | 15–30 hours | 3 mg |
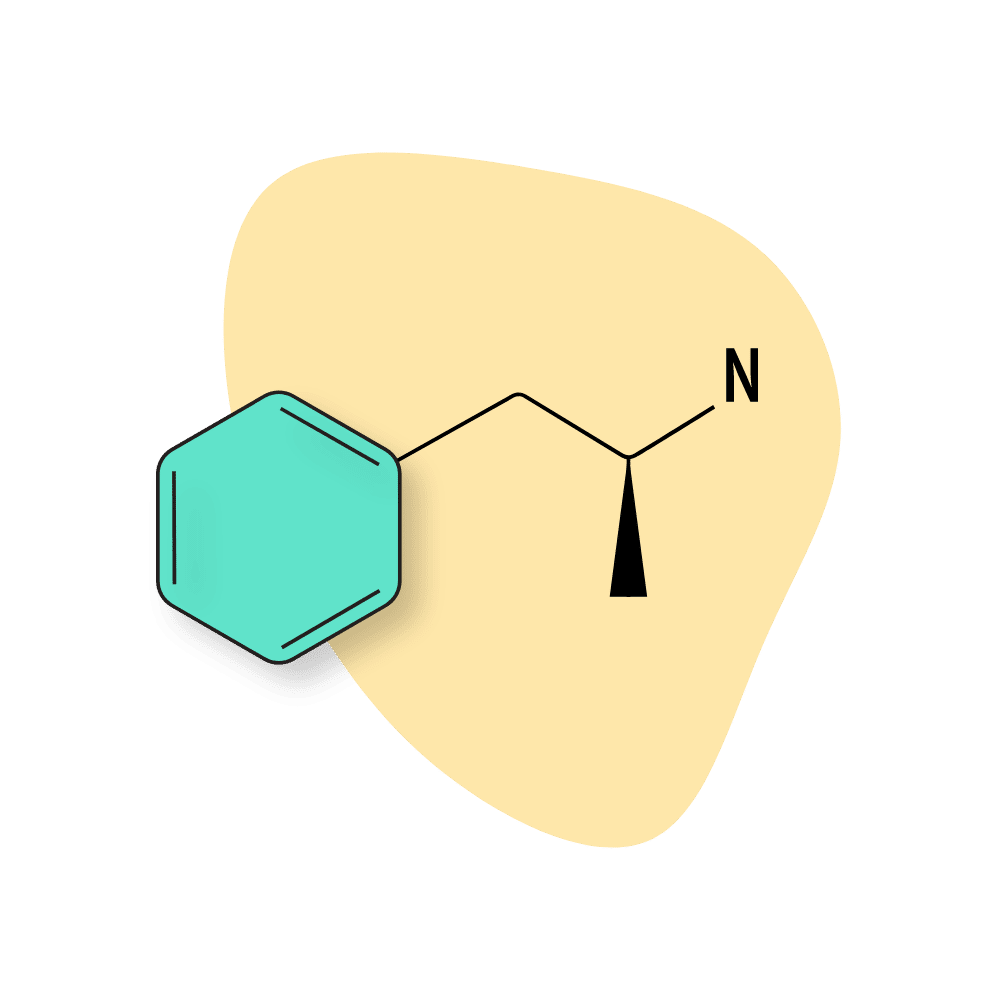
| Amphetamine | Approved 💊 | 4–8 hours | 5 mg |
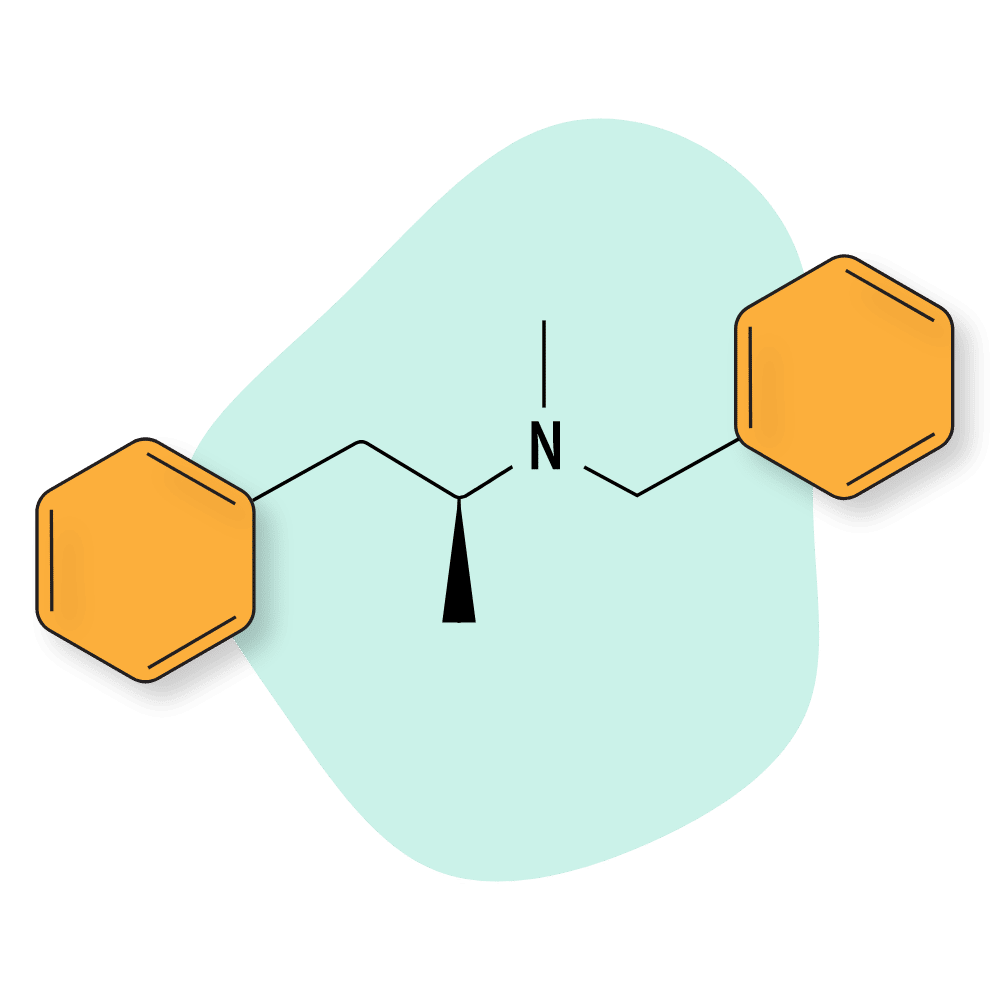
| Benzphetamine | Approved 💊 | 3–5 hours | 15 mg |
| Buphedrone | RC 🧪 | 2–5 hours | 10 mg |
| Bupropion | Approved 💊 | 2–3 hours | 100 mg |
| Butylone | RC 🧪 | 3–5 hours | 20 mg |
| Cathinone | RC 🧪 | 3–5 hours | 5 mg |
| DOAM | RC 🧪 | Unknown | 5 mg |
| DOB | RC 🧪 | 8–24 hours | 0.25 mg |
| DOBU | RC 🧪 | Unknown (Long) | Unknown |
| DOC | RC 🧪 | 10–20 hours | 0.5 mg |
| DOEF | RC 🧪 | 12–16 hours | 1 mg |
| DOET | RC 🧪 | 12–30 hours | 1 mg |
| DOI | RC 🧪 | 12–18 hours | 0.7 mg |
| DOM | RC 🧪 | 8–30 hours | 0.5 mg |
| DON | RC 🧪 | 14–22 hours | 2 mg |
| DOPR | RC 🧪 | 18–30 hours | 1.25 mg |
| Ethcathinone (ETH-CAT) | RC 🧪 | 3–5 hours | 50 mg |
| Ethylone | RC 🧪 | 2–4 hours | 50 mg |
| Lisdexamfetamine | Approved 💊 | 10–14 hours | 10 mg |
| MDA | Approved 💊 | 5–9 hours | 40 mg |
| MDMA | RC 🧪 | 4–6 hours | 40 mg |
| MDPH | RC 🧪 | 3–5 hours | 100 mg |
| MDPV | RC 🧪 | 2–7 hours | 2 mg |
| MEM | RC 🧪 | 8–16 hours | 15 mg |
| Mephedrone (4-MMC) | RC 🧪 | 2–3 hours | 25 mg |
| Methamphetamine | RC 🧪 | 10–12 hours | 2.5 mg |
| Methylone | RC 🧪 | 2.5–4 hours | 75 mg |
| MMDA | RC 🧪 | 5–7 hours | 50 mg |
| N-Ethylamphetamine | Approved 💊 | 6–12 hours | 5 mg |
| N,N-Dimethylamphetamine | Approved 💊 | 12–24 hours | 5 mg |
| PMA | RC 🧪 | 1–2 hours | 10 mg |
| PMMA | RC 🧪 | 1–2 hours | 30 mg |
| Propylamphetamine | RC 🧪 | Unknown | Unknown |
| Selegiline | Approved 💊 | 2–5 hours | 5 mg |
| TMA-2 | RC 🧪 | 6–12 hours | 10 mg |
| TMA-6 | RC 🧪 | 10–18 hours | 10 mg |
| Xylopropamine | Approved 💊 | 1–3 hours | 5 mg |
 | α-PPP | RC 🧪 | 1–3 hours | 1 mg |
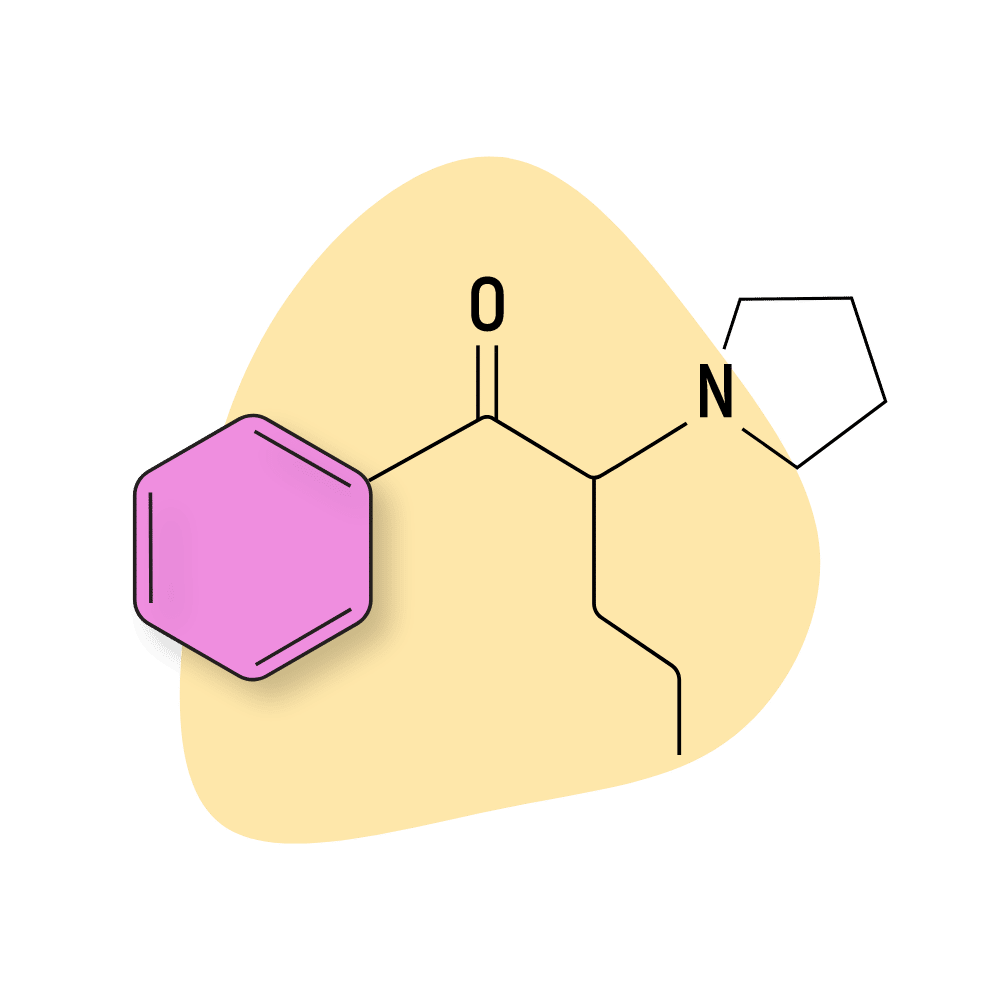 | α-PVP | RC 🧪 | 1–3 hours | 1 mg |
Basic Amphetamines
Amphetamines have been around for a long time — the first one (amphetamine itself) was discovered over 130 years ago. Throughout the 1900s, new amphetamine derivatives and prodrugs were invented in an attempt to create new medications.
Most of the members in this group are psychoactive but not psychedelic. They offer varying levels of stimulating, entactogenic, and euphoric effects.
The most well-known members of this group are amphetamine (Adderall), lisdexamfetamine (Vyvanse), and N-Methamphetamine (crystal meth).
4-ETA
4-ETA (AKA para-ethoxyamphetamine) is a close relative of the more well-known PMA (para-methoxyamphetamine). 4-ETA is gentler than PMA but still produces all the negative aspects of MDMA (tachycardia, dehydration, hyperthermia) without any of the desirable parts (the euphoria, increased empathy, and love, and laughter).
This is not a compound people tend to take on purpose. Anything more than small doses quickly becomes uncomfortable and it doesn’t take much to overdose.
The effects of 4-ETA are comparable but noticeably gentler than PMA, which has garnered a bad reputation for being too stimulating — frequently leading to side effects like hyperthermia, dehydration, tachycardia, and anxiety.
4-ETA Specs:
| Chemical Name: | Para-Ethoxyamphetamine |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 10–14 hours |
| Estimated Threshold Dose: | 10 mg |
| Common Dose: | 20–50 mg |
| PubChem ID: | 162223124 |
| CAS#: | 129476-58-0 |

IUPAC NAME: 1-(4-ethoxyphenyl)propan-2-amine
Amphetamine (Adderall)
Amphetamine is the base molecule from which all others in this class are based. It was first discovered 136 years ago in Germany (1887).
The chemical structure of amphetamine closely resembles dopamine and norepinephrine. As such, amphetamine works by interacting with the receptors for these neurotransmitters in different ways.
The effects of amphetamine are primarily stimulating. Depending on the dose, it may also have euphoriant, aphrodisiac, and decongestant properties.
Numerous medications available on the market today use amphetamine as one of the active ingredients. Some of the most common include Adderall, Dynavel, Evekeo, Zenzedi (dextroamphetamine), and others.
There are two enantiomers of amphetamine — levoamphetamine (l-amphetamine) and dextroamphetamine (d-amphetamine). D-amphetamine has a higher impact on norepinephrine than l-amphetamine.
Many other basic amphetamines offer comparable effects to amphetamine, including lisdexamfetamine, methamphetamine, or other stimulants such as modafinil or methylphenidate.
Amphetamine Specs:
| Chemical Name: | Amphetamine |
| Status: | Approved |
| Duration of Effects: | 6–12 hours |
| Estimated Threshold Dose: | 2 mg |
| Common Dose: | 20–50 mg |
| PubChem ID: | 3007 |
| CAS#: | 300-62-9 |

IUPAC Name: 1-phenylpropan-2-amine
Benzphetamine (Didrex)
Benzphetamine is a substituted amphetamine sold under the brand name Didrex. It’s most commonly used as a prescription weight loss medication (rarely used for recreational use).
Benzphetamine works as a prodrug for both dextroamphetamine and dextromethamphetamine. It has potent anti-appetite effects and mild stimulating qualities.
Benzphetamine Specs:
| Chemical Name: | Benzphetamine |
| Status: | Approved |
| Duration of Effects: | 3–5 hours |
| Estimated Threshold Dose: | 10 mg |
| Common Dose: | 25–100 mg |
| PubChem ID: | 5311017 |
| CAS#: | 156-08-1 |

IUPAC Name: (2S)-N-benzyl-N-methyl-1-phenylpropan-2-amine
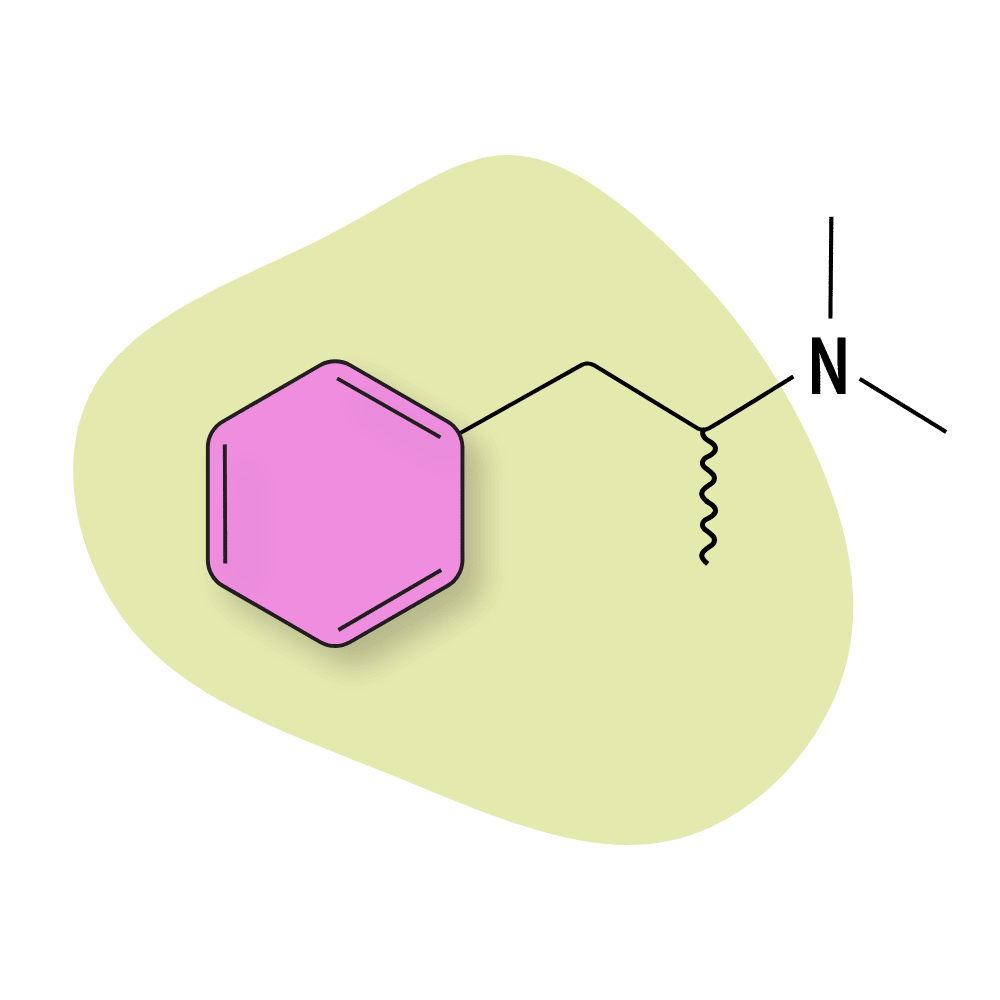
Dimethylamphetamine
Dimethylamphetamine (N,N-Dimethylamphetamine) was briefly sold under the brand name Metrotonin but is now listed as a Schedule I restricted substance.
This compound acts as a prodrug for both amphetamine and methamphetamine [18]. It initially sparked some interest in the 90s as a potentially less addictive and longer-lasting version of its more popular active metabolites — however, due to the much lower potency of this compound (6–12 times less potent than methamphetamine), it never caught on as a viable alternative to either compounds.
Dimethylamphetamine Specs:
| Chemical Name: | N,N-Dimethylamphetamine |
| Status: | Approved (Now Banned) |
| Duration of Effects: | 12–24 hours |
| Estimated Threshold Dose: | 5 mg |
| Common Dose: | 10–30 mg |
| PubChem ID: | 20006 |
| CAS#: | 4075-96-1 |

IUPAC Name: N,N-dimethyl-1-phenylpropan-2-amine
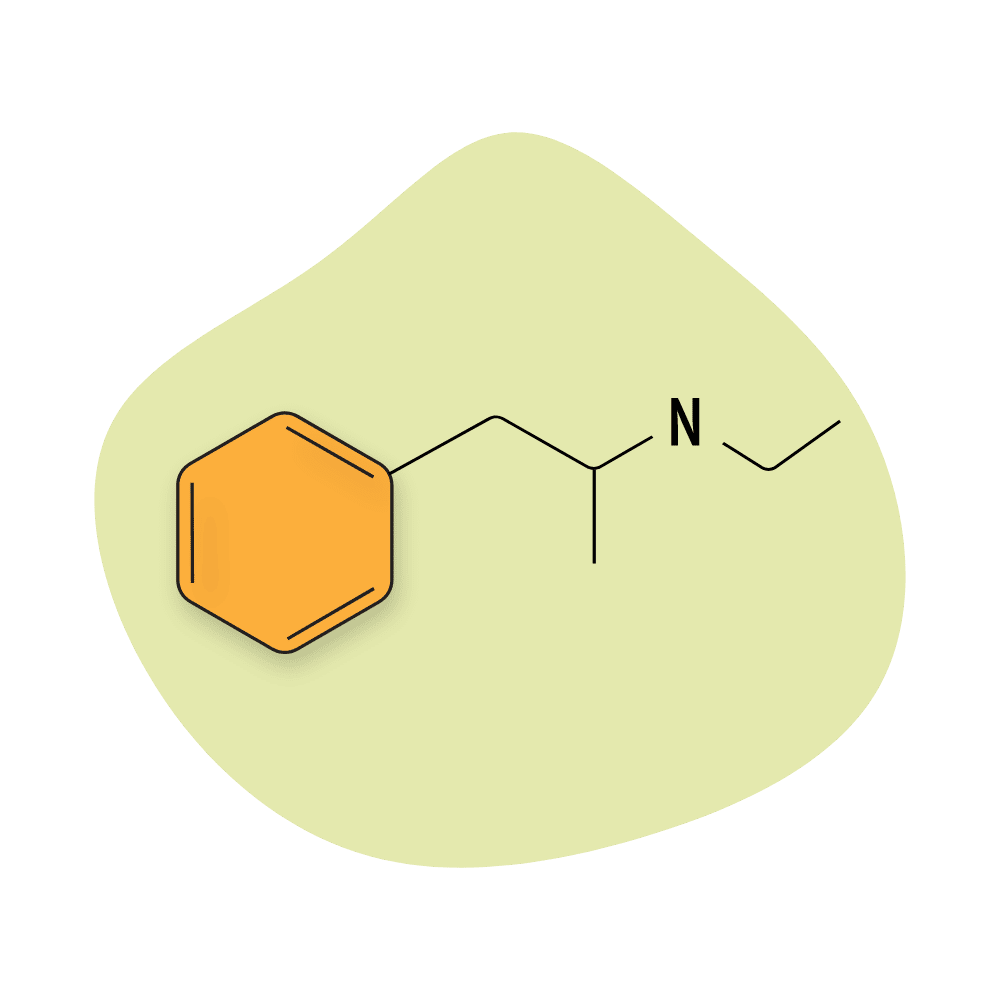
Ethylamphetamine
Ethylamphetamine (AKA etilamfetamine) is a prescription drug sold under the trade names Apetinil and Adiparthrol. It’s prescribed as a weight loss treatment due to its stimulating and appetite-suppressant qualities.
There are some people who use this drug recreationally as an alternative to methamphetamine, but the effects of N-ethylamphetamine are milder and less euphoric.
The chemical structure of ethylamphetamine is just one carbon away from methamphetamine.
Ethylamphetamine Specs:
| Chemical Name: | N-Ethylamphetamine |
| Status: | Approved |
| Duration of Effects: | 6–12 hours |
| Estimated Threshold Dose: | 5 mg |
| Common Dose: | 10-30mg |
| PubChem ID: | 9982 |
| CAS#: | 457-87-4 |

IUPAC Name: N-ethyl-1-phenylpropan-2-amine
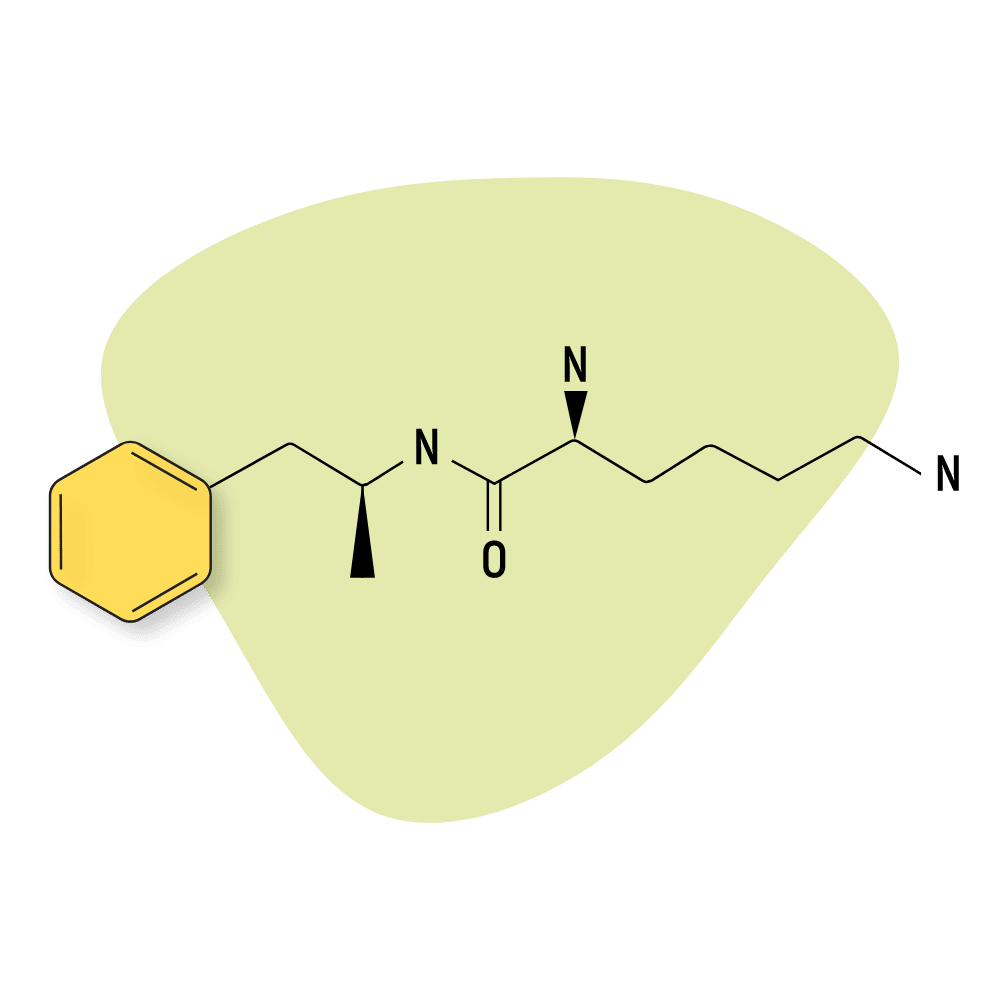
Lisdexamfetamine (Vyvanse)
Lisdexamfetamine is better known under its trade name, Vyvanse. It’s used as a treatment for attention deficit hyperactivity disorder (ADHD), binge-eating disorder, and off-label treatment for narcolepsy. Like many basic amphetamines, Vyvanse is a prodrug for dextroamphetamine, which is what exerts the bulk of this medication’s stimulating effects.
This drug is rarely used recreationally but is often used as a sort of nootropic to improve focus and attention while studying or working. Because of its medical value, Vyvanse is listed as a Schedule II prescription-only medication in the US and Canada and as a Class B drug in the UK.
Related: An ADHD Conspiracy Why Your Vyvanse May Not Be Working
Lisdexamfetamine Specs:
| Chemical Name: | Lisdexamfetamine |
| Status: | Approved |
| Duration of Effects: | 10–14 hours |
| Estimated Threshold Dose: | 10 mg |
| Common Dose: | 20–30 mg |
| PubChem ID: | 11597698 |
| CAS#: | 608137-32-2 |

IUPAC Name: (2S)-2,6-diamino-N-[(2S)-1-phenylpropan-2-yl]hexanamide
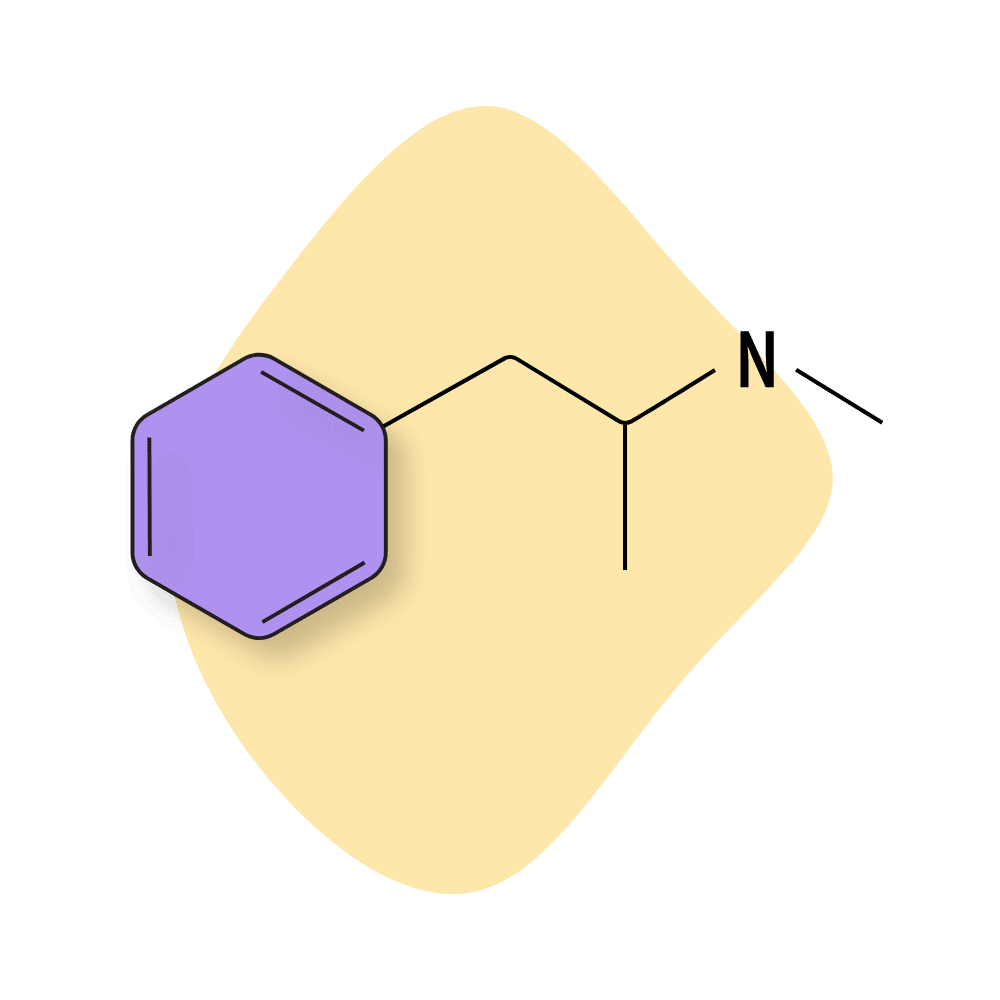
Methamphetamine (Meth)
N-methamphetamine (AKA Meth, Ice, Yaba, and others) is a common recreational drug. It’s cheap to make and provides desirable effects like euphoria and increased energy. This compound is very similar to amphetamine but can cross the blood-brain barrier much more quickly due to improvements in lipid solubility. Meth can be taken orally, snorted, smoked, or injected.
Meth use is especially associated with sexual activity — it increases sexual desire, enhances stimulation, and delays ejaculation. This combo, mixed with meth’s effects on mood, inhibition, and energy, has led to the formation of an entire subculture of “party and play” (PnP) or “chemsex” — by which participants will use meth and have sex over and over for several days on end.
Methamphetamine interacts with several key neurotransmitters, including dopamine, serotonin, and norepinephrine.
Methamphetamine Specs:
| Chemical Name: | Methamphetamine |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 10–20 hours |
| Estimated Threshold Dose (Oral): | 5 mg |
| Common Dose (Oral): | 10–30 mg |
| PubChem ID: | 10836 |
| CAS#: | 537-46-2 |

IUPAC Name: (2S)-N-methyl-1-phenylpropan-2-amine
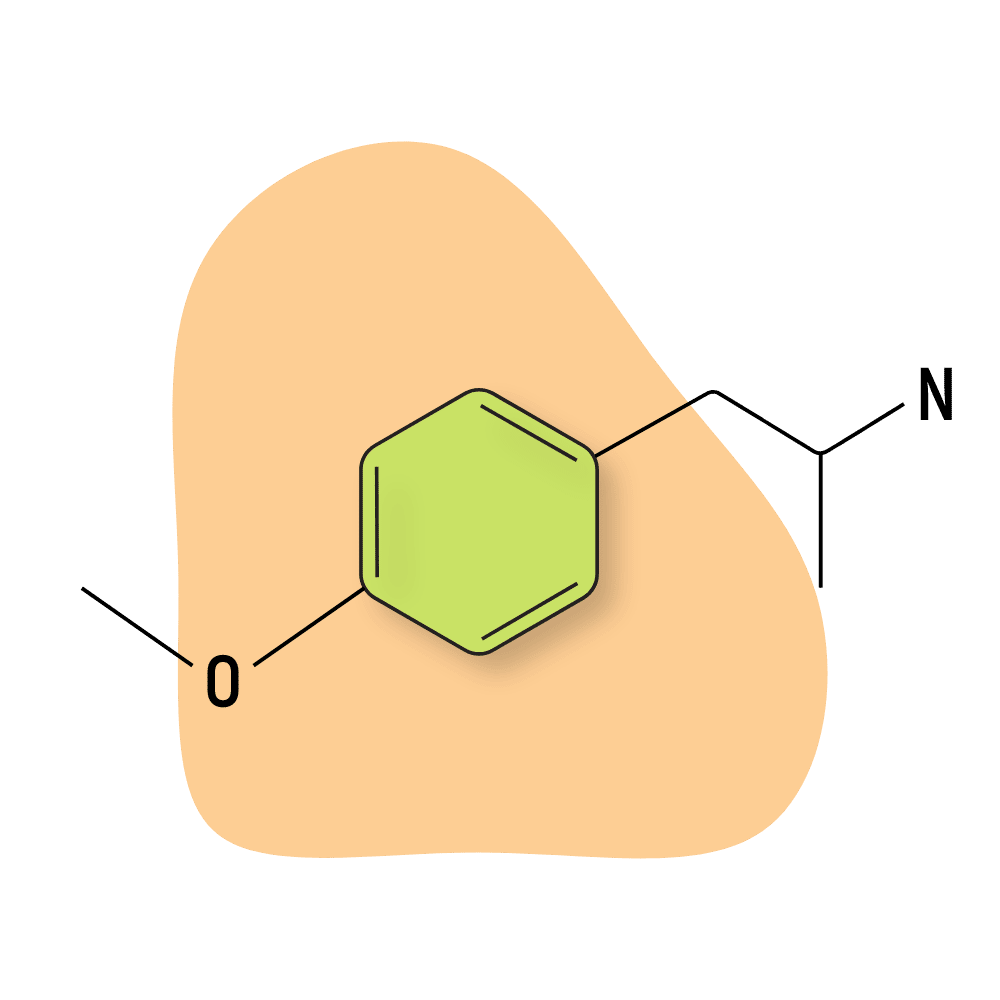
PMA (Para-Methoxyamphetamine)
PMA (para-methoxyamphetamine) — sometimes known as 4-methoxyamphetamine or 4-MeOA — has very little value as a recreational drug. It has minor entactogenic effects due to the serotonergic effects, but the threshold between a psychoactive dose and an overdose is very narrow.
PMA is not a drug of choice by any means but is instead used as an adulterant for MDMA or MDA. PMA is dangerous and has led to many deaths over the years. The lethal dose of PMA is below the standard dose of MDMA, so a single capsule of pure PMA mistaken for MDMA could be lethal.
PMA or PMMA overdoses usually result when people take what they believe to be ecstasy, and when they don’t feel strong effects right away, they take more and end up overdosing on PMA or PMMA instead.
The effects of PMA go from 0 to 100 in an instant — causing severe hyperthermia, hypertension, arrhythmia, and dizziness.
This substance should not be used for any reason. It’s a good example of why testing unknown drugs before taking them is so important.
PMA Specs:
| Chemical Name: | 4-Methoxyamphetamine |
| Status: | Not Used |
| Duration of Effects: | 6–12 hours |
| Estimated Threshold Dose: | Not Used |
| Common Dose: | 30–60 mg (Not Recommended) |
| PubChem ID: | 31721 |
| CAS#: | 64-13-1 |

IUPAC Name: 1-(4-methoxyphenyl)propan-2-amine
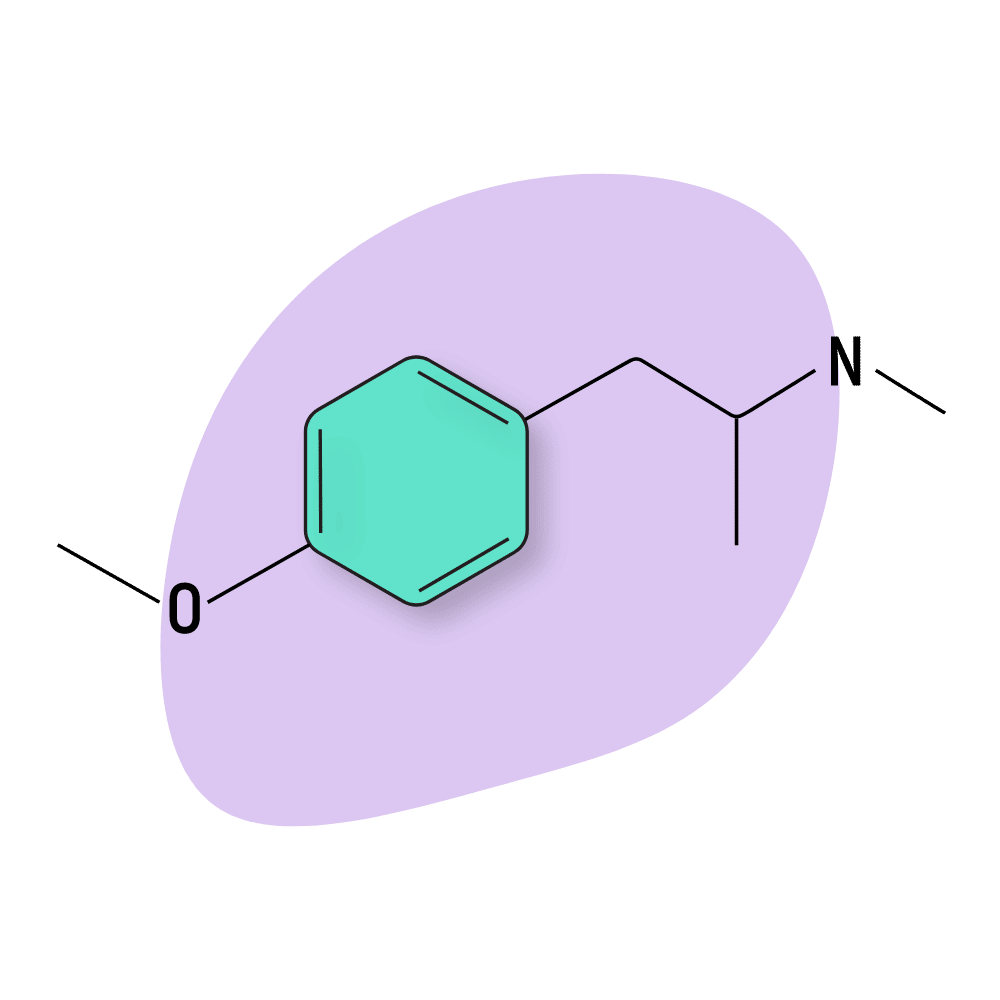
PMMA (Para-Methoxymethamphetamine)
PMMA (para-methoxymethamphetamine) is another dangerous amphetamine substitute most similar to PMA (albeit slightly less potent).
Both PMMA and PMA work as selective serotonin-releasing agents (SSRAs). This, combined with their selectivity for the MAO-A receptor (low to no activity at MAO-B), is believed to be responsible for the toxic effects of both PMMA and PMA. The symptoms of overdose strongly correlate with serotonin syndrome, which is caused by a toxic buildup of serotonin in the central nervous system, leading to lethal and potentially irreversible damage to the central nervous system.
Users who take PMMA (or relatives including PMA and 4-ETA) experience side effects including hyperthermia (the most common cause of death from using this substance), seizures, delirium, tremors, hypertension, and tachycardia.
It’s important to test all amphetamine psychedelics for PMA and PMMA prior to use. If these compounds are detected, the sample should be disposed of immediately. PMMA offers no recreational value and can be lethal.
PMMA Specs:
| Chemical Name: | 4-Methoxymethamphetamine |
| Status: | Not Used |
| Duration of Effects: | 6–12 hours |
| Estimated Threshold Dose | Not Used |
| Common Dose: | 80–100 mg (Not Recommended) |
| PubChem ID: | 90766 |
| CAS#: | 22331-70-0 |

IUPAC Name: 1-(4-methoxyphenyl)-N-methylpropan-2-amine
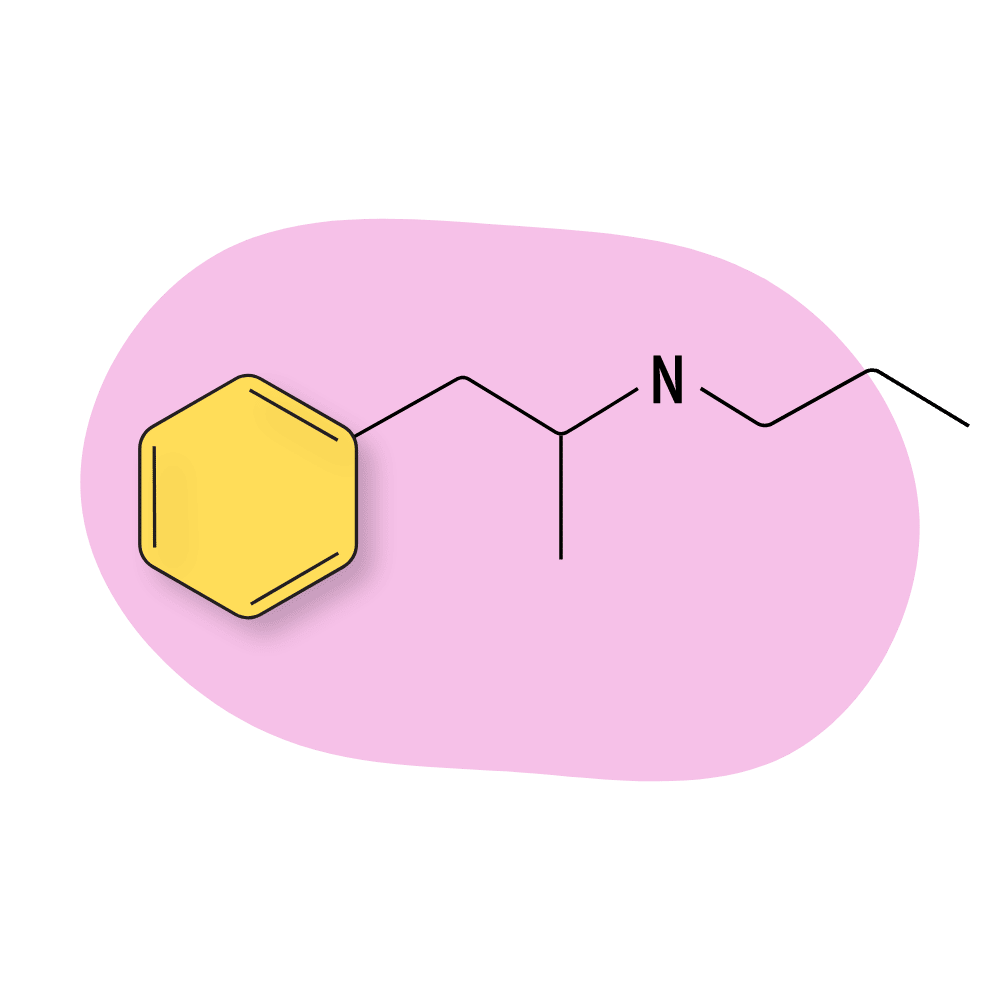
Propylamphetamine
Propylamphetamine isn’t used much today. It was developed in the 1970s as a prodrug for amphetamine but never caught on in practice because it’s much less potent than amphetamine itself and doesn’t offer any advantages in terms of cost, potency, or effect profile.
Propylamphetamine Specs:
| Chemical Name: | Propylamphetamine |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | Unknown |
| Estimated Threshold Dose: | Unknown |
| Common Dose: | Unknown |
| PubChem ID: | 103544 |
| CAS#: | 51799-32-7 |

IUPAC Name: 1-phenyl-N-propylpropan-2-amine
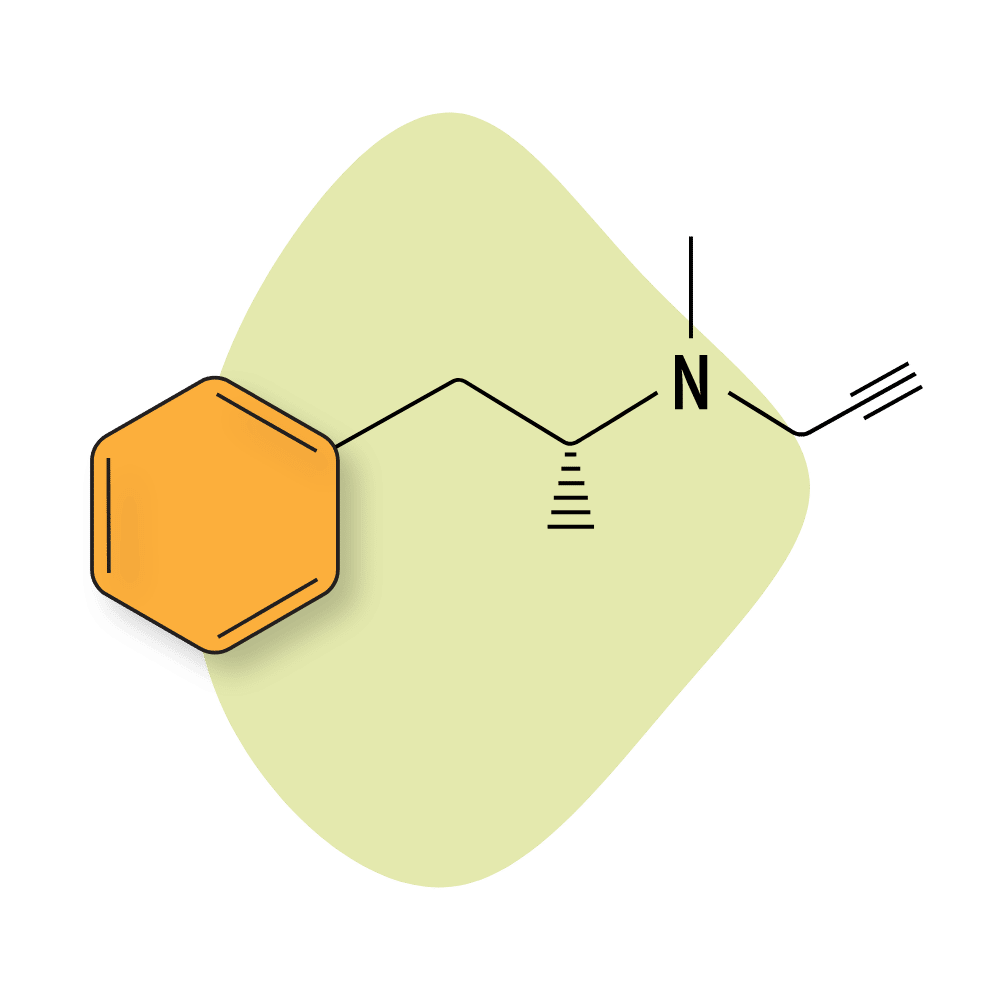
Selegiline
Selegiline (AKA L-Deprenyl) is a prescription medication sold under the trade names Eldepryl and Emsam. It’s a monoamine inhibitor (MAO-B) used for treating Parkinson’s disease and major depressive disorder. MAO-B inhibitors lead to an increase in dopamine in the brain.
There isn’t much interest in this compound as a designer drug on its own, but it’s sometimes mixed with other amphetamines to boost their effects.
Selegiline Specs:
| Chemical Name: | Selegiline |
| Status: | Approved |
| Duration of Effects: | 2–5 hours |
| Estimated Threshold Dose: | 5 mg |
| Common Dose: | 10–20 mg |
| PubChem ID: | 26757 |
| CAS#: | 14611-51-9 |

IUPAC Name: (2R)-N-methyl-1-phenyl-N-prop-2-ynylpropan-2-amine
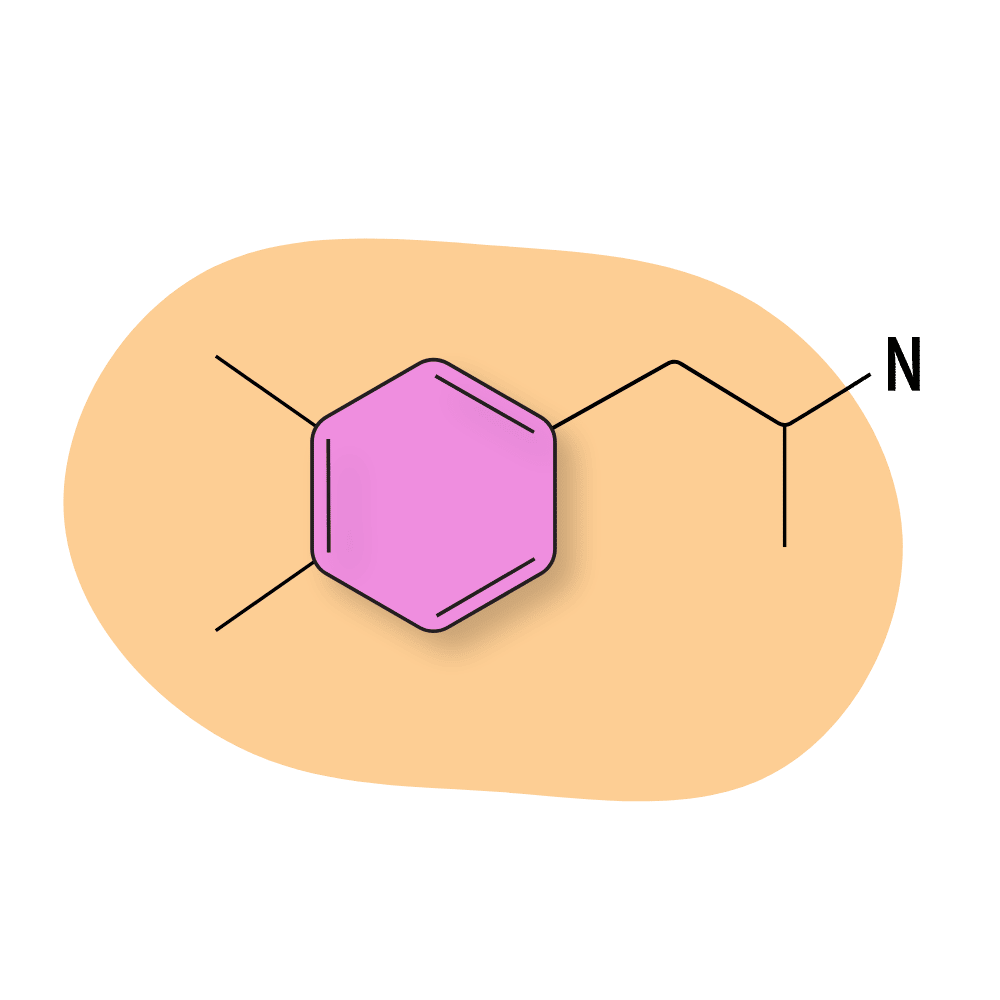
Xylopropamine
Xylopropamine is sold under the trade names Perhedrin and Esanin. It was used as an appetite suppressant and weight-loss medication in the 1950s but is no longer in use today because of its high risk of side effects and the existence of other, safer medications.
This compound is sometimes used recreationally but tends to be combined with other drugs to offset its negative effects (like muscle tremors or panic attacks).
Some of the early research on this compound proposed it could be useful as an amphetamine-based analgesic, but this research was never completed [19].
Xylopropamine Specs:
| Chemical Name: | Xylopropamine |
| Status: | Approved |
| Duration of Effects: | 1–3 hours |
| Estimated Threshold Dose: | 5 mg |
| Common Dose: | 10–20 mg |
| PubChem ID: | 26727 |
| CAS#: | 102-31-8 |

IUPAC NAME: 1-(3,4-dimethylphenyl)propan-2-amine
Fluorinated Amphetamines
The fluorinated amphetamines first started appearing on the market around 2010. The most common members of this group include 2-FMA, 2-FA, and 4-FA.
These are characterized by a fluoride group attached to the central ring moiety of the base amphetamine molecule. The addition of fluorine significantly alters the bioavailability and selectivity of a substance on various receptors — often unpredictably.
Unfortunately, fluorinated substances also release radioactive fluoride atoms into the brain, which can cause long-term cognitive problems.
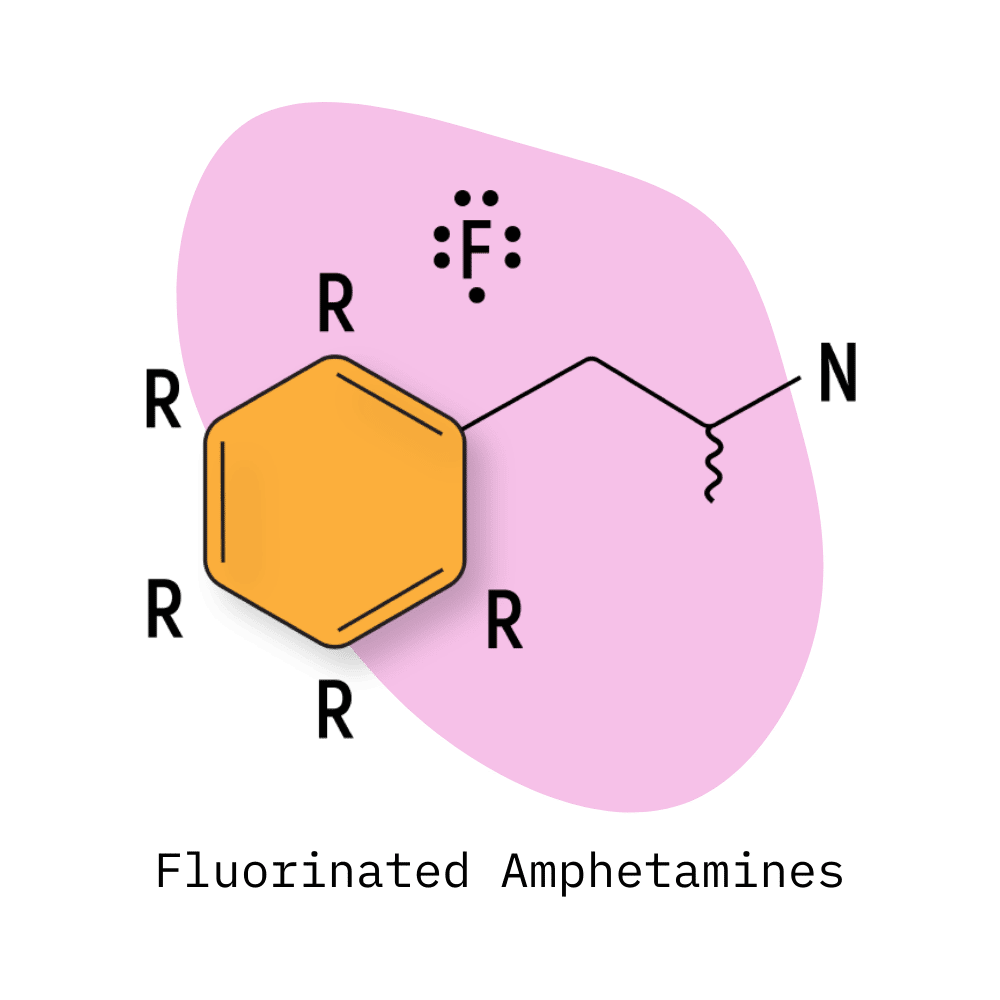
2-FA, 2-FMA, and 2-FEA contain fluoride at the 2-position. 3-FA, 3-FMA, and 3-FEA feature this fluoride at the 3-position. 4-FA and 4-FMA feature the fluoride at the 4-position.
Most of the members of this group are believed to act as dopamine and norepinephrine-releasing agents — but very little research is available for these compounds to date despite their widespread popularity in the designer drug space.
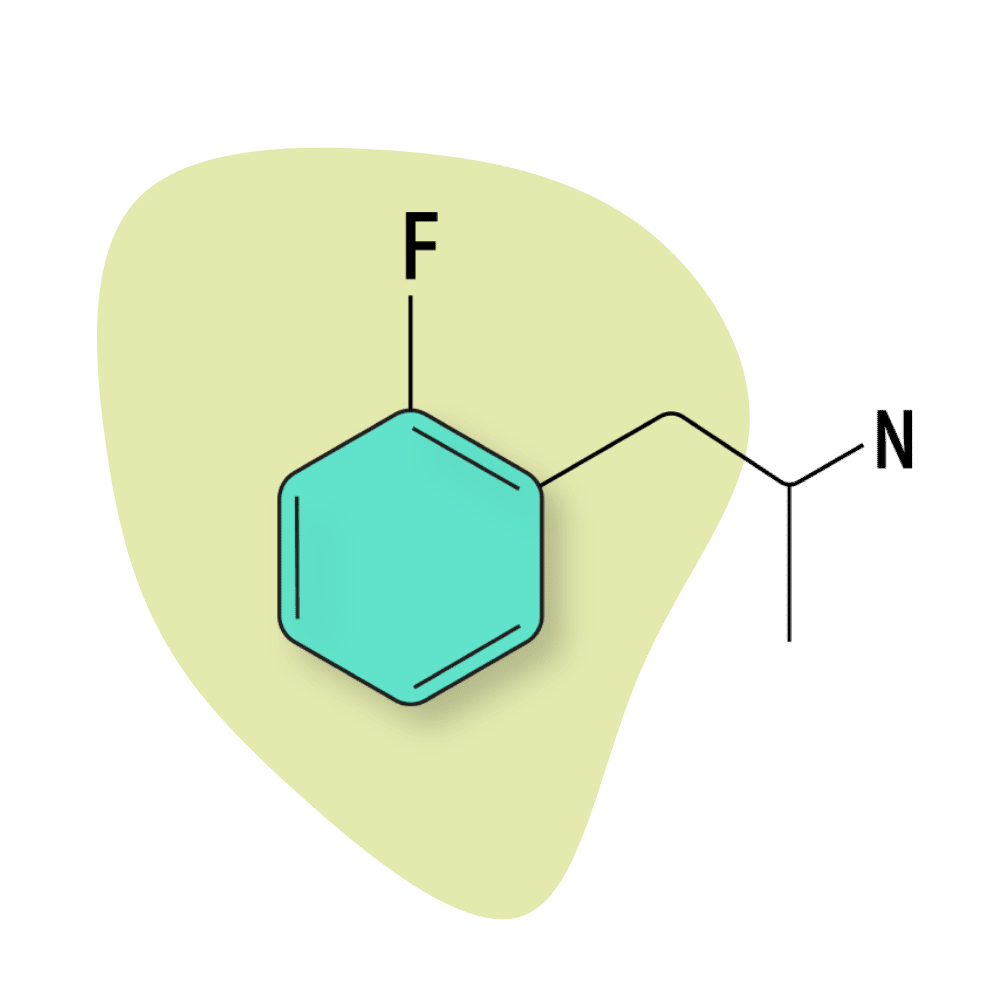
2-FA
2-FA is considered to be the closest in terms of effect profile to amphetamine. It’s a strong stimulant, with the threshold dose listed somewhere around 5 mg and the standard psychoactive dose around 30 mg.
As far as fluorinated amphetamines go, 2-FA is reported to have the lowest risk for unwanted side effects. However, it’s easy to take too much, leading to significant discomfort in the form of nausea, dizziness, anxiety, and stomach pain.
This compound can be insufflated (snorted) or vaped, but these modes of administration often lead to uncomfortable side effects.
2-FA Specs:
| Chemical Name: | 2-Fluoroamphetamine |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 2–4 hours |
| Estimated Threshold Dose: | 5 mg |
| Common Dose: | 20–30 mg |
| PubChem ID: | 121531 |
| CAS#: | 1716-60-5 |

IUPAC Name: 1-(2-fluorophenyl)propan-2-amine
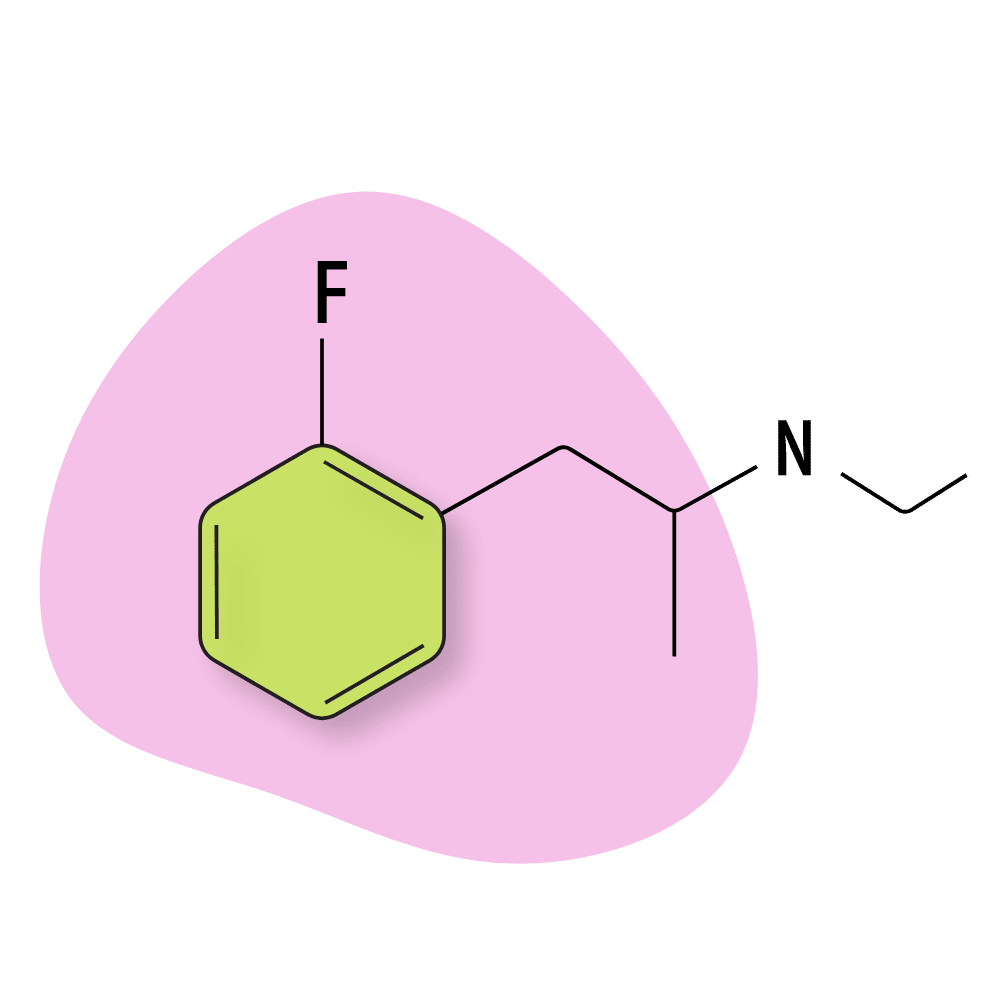
2-FEA
Little is known about 2-FEA, but from the few user accounts online, it appears the effects are more serotonergic than dopaminergic (like most fluorinated amphetamines). The effects are primarily euphoric, mildly hallucinogenic (tracers), and highly stimulating.
This molecule is believed to act as a triple-reuptake inhibitor — blocking the reuptake of serotonin, dopamine, and norepinephrine.
2-FEA Specs:
| Chemical Name: | 2-Fluorethamphetamine |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 1–2 hours |
| Estimated Threshold Dose: | 15 mg |
| Common Dose: | 20–30 mg |
| PubChem ID: | 20027466 |
| CAS#: | 3823-29-8 |

IUPAC Name: N-ethyl-1-(2-fluorophenyl)propan-2-amine
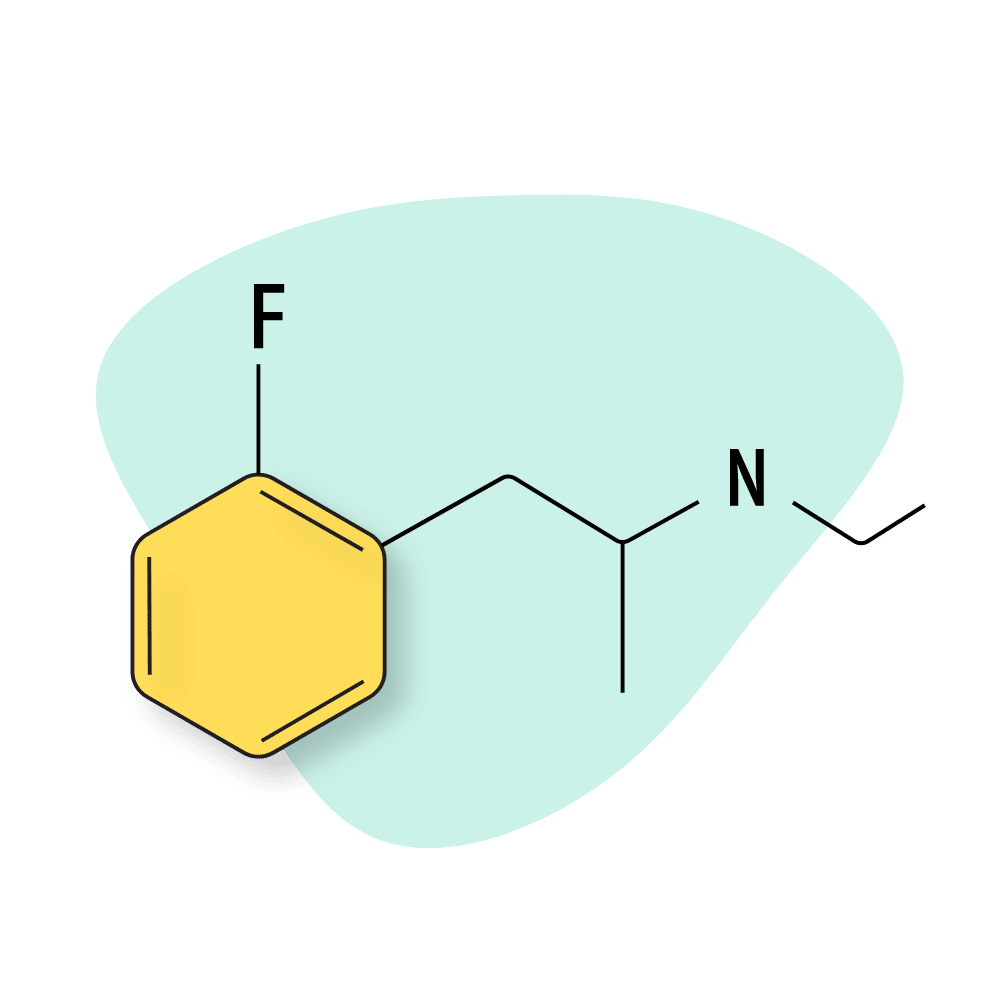
2-FMA
2-FMA is a structural analog of methamphetamine and shares similar effects. It’s taken orally or through insufflation (snorting) but should never be vaped or smoked. Applying too much heat releases free fluoride, which is toxic.
This long-lasting fluorinated amphetamine is less popular than 2-FA for recreational use but is more commonly used as a productivity or study aid. The effects are said to be most comparable to Vyvanse (lisdexamfetamine) in this regard.
2-FMA Specs:
| Chemical Name: | 2-Fluoromethamphetamine |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 7–9 hours |
| Estimated Threshold Dose: | 10 mg |
| Common Dose: | 25–35 mg |
| PubChem ID: | 24257263 |
| CAS#: | 1017176-48-5 |

IUPAC Name: 1-(2-fluorophenyl)-N-methylpropan-2-amine
3-FA
3-FA is regarded as a milder form of methamphetamine. Everybody reacts differently to this substance — some claim it produces euphoria and a strong spacey sensation; others claim it’s just a weak form of meth. There isn’t enough anecdotal data and virtually no clinical research on this compound to work out an effective dose.
With that said, the common dose range appears to sit somewhere between 20 and 200 mg, with some users taking as much as 300 mg in a single session.
3-FA Specs:
| Chemical Name: | 3-Fluoroamphetamine |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 4–6 hours |
| Estimated Threshold Dose: | 10 mg |
| Common Dose: | 15–30 mg |
| PubChem ID: | 121501 |
| CAS#: | 1626-71-7 |

IUPAC Name: 1-(3-fluorophenyl)propan-2-amine
3-FMA
3-FMA is one of the newer additions to the fluorinated amphetamine group. It’s considered to have a stronger entactogenic and empathogenic effect profile than most of the other fluorinated amphetamines.
While there are no official pharmacological studies on this compound, it’s believed to act as a serotonin-dominant reuptake inhibitor, which could explain the more empathogenic and entactogen effects reported by users online.
3-FMA Specs:
| Chemical Name: | 3-Fluoromethamphetamine |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 3–7 hours |
| Estimated Threshold Dose: | 5 mg |
| Common Dose: | 15–30 mg |
| PubChem ID: | 58216164 |
| CAS#: | 1182818-14-9 |

IUPAC Name: 1-(3-fluorophenyl)-N-methylpropan-2-amine
3-FEA
The effects of 3-FEA are most comparable to 4-FA, with potent stimulating and mild entactogenic qualities. This compound still isn’t well-known and has only been on the market since the late 2010s.
Most users report this drug to be a bit underwhelming compared to many other compounds in the amphetamine family. It’s stimulating in higher doses but carries a mental “heaviness” that can make users feel couch-locked.
3-FEA Specs:
| Chemical Name: | 3-Fluoroethamphetamine |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 4–6 hours |
| Estimated Threshold Dose: | 15 mg |
| Common Dose: | 50–150 mg |
| PubChem ID: | 57458869 |
| CAS#: | 725676-94-8 |
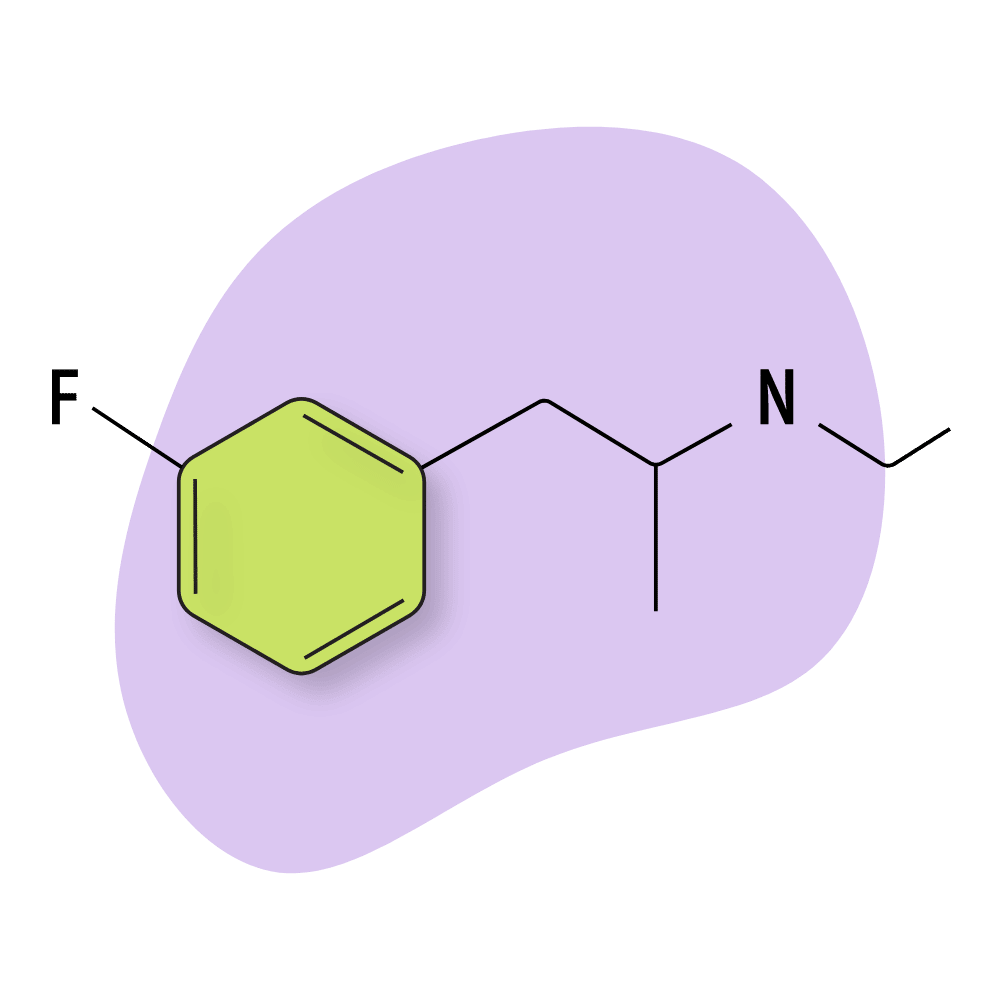
IUPAC Name: N-ethyl-1-(3-fluorophenyl)propan-2-amine
3-FPM
3-FPM is a derivative of phenmetrazine, a stimulant medication used in the 1950s. The effects of 3-FPM are similar to amphetamine, though slightly less potent. Some people take this RC as a functional stimulant to help with focus and motivation while working or studying.
The most common method of using this substance is by snorting, but the powder tends to cause a lot of irritation in the nose and throat. Tolerance also appears to form quickly.
3-FPM Specs:
| Chemical Name: | 3-Fluorophenmetrazine |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 4–6 hours |
| Estimated Threshold Dose: | 10 mg |
| Common Dose: | 40–80 mg |
| PubChem ID: | 54673723 |
| CAS#: | 1350768-28-3 |
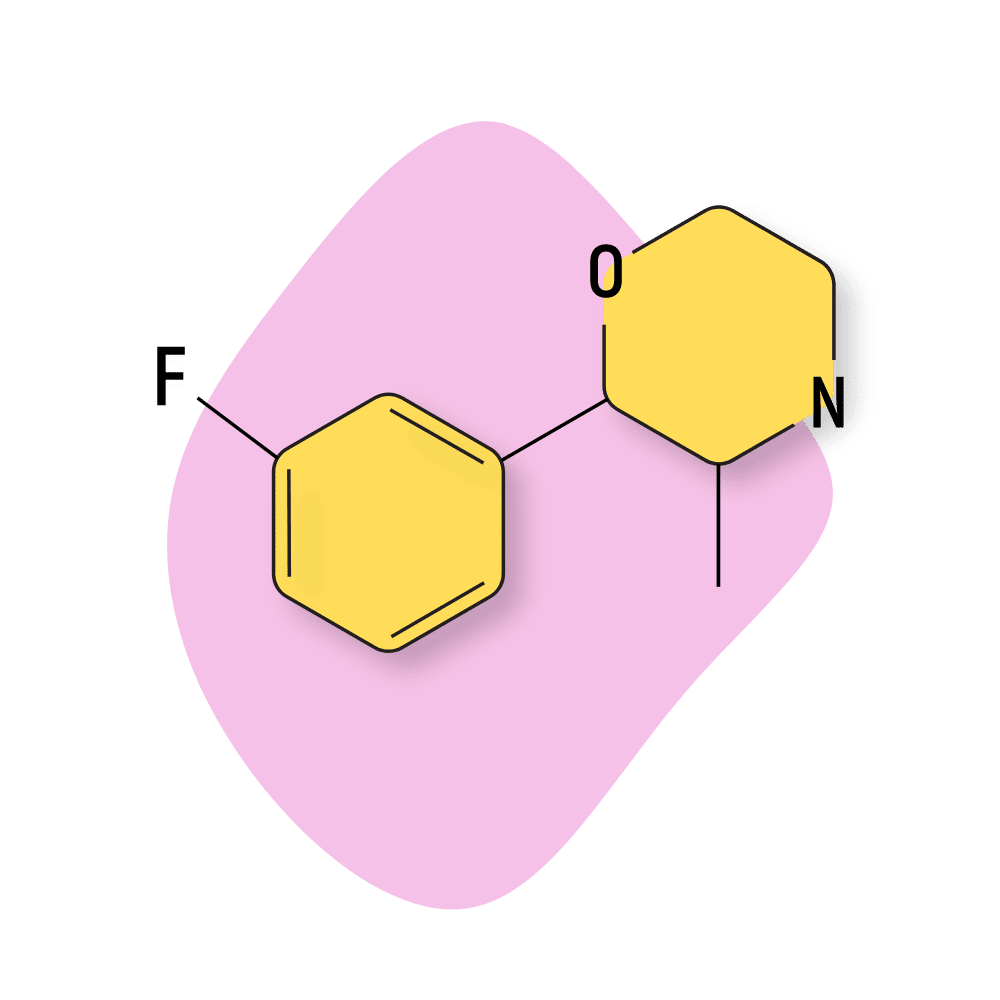
IUPAC Name: 2-(3-fluorophenyl)-3-methylmorpholine
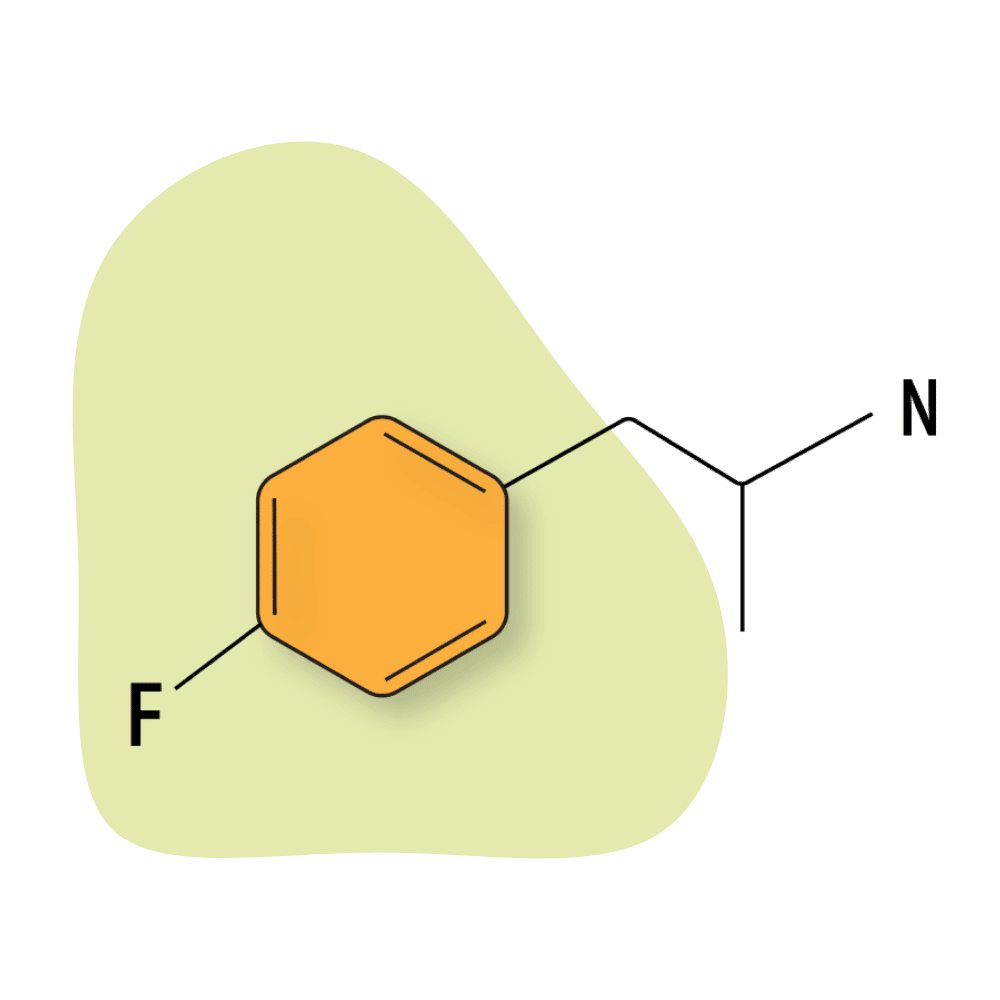
4-FA
4-FA is sometimes sold under the name PAL-303 or Flux. It’s one of the more popular members of the fluorinated amphetamine class.
The effects of this compound are distinct from other members of this group. It’s long-lasting, and the effects tend to wax and wane over the course of a few hours. First, 4-FA feels strongly euphoric and empathogenic before transitioning into the classic stimulating profile of amphetamine for the second half of the experience.
This compound is also strongly associated with sexual stimulation in a similar capacity to methamphetamine but without social extroversion. 4-FA is more of a solo drug.
4-FA Specs:
| Chemical Name: | 4-Fluoroamphetamine |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 5–8 hours |
| Estimated Threshold Dose: | 40 mg |
| Common Dose: | 75–125 mg |
| PubChem ID: | 9986 |
| CAS#: | 459-02-9 |

IUPAC Name: 1-(4-fluorophenyl)propan-2-amine
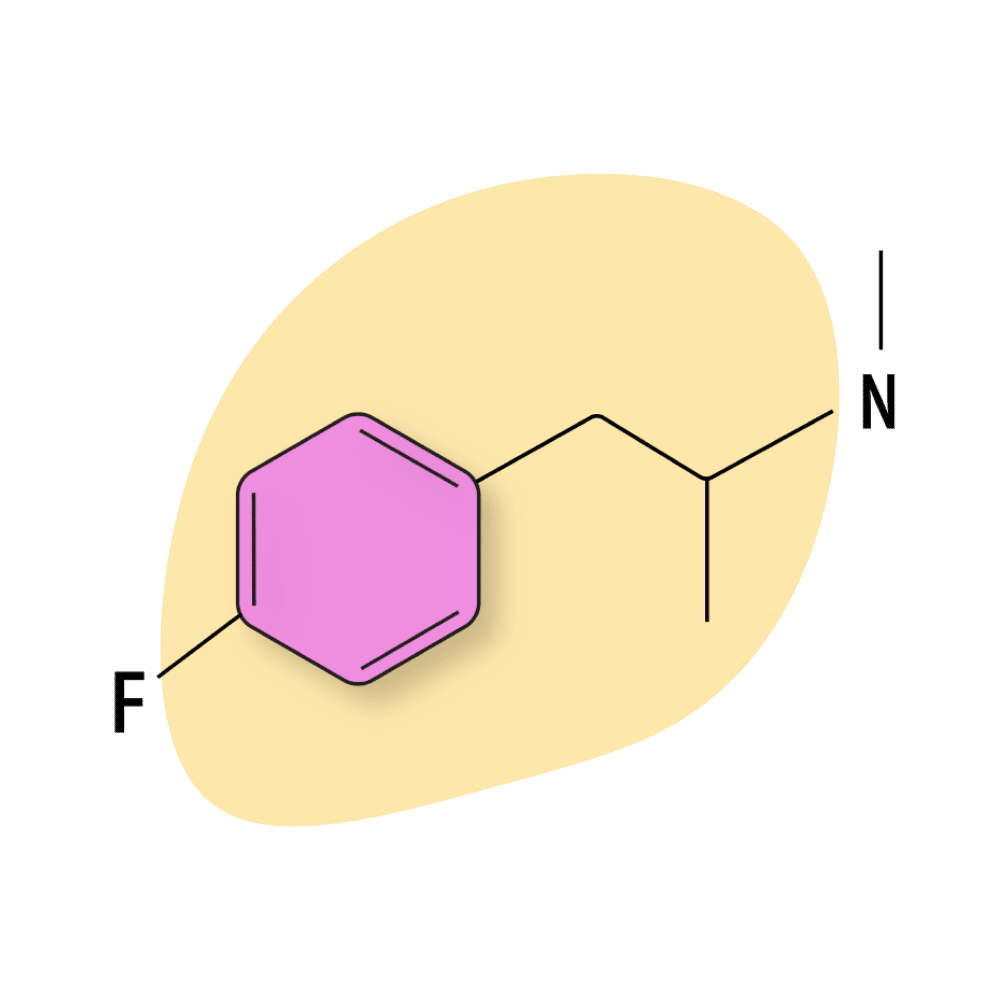
4-FMA
4-FMA is less popular than 4-FA because of the heightened risk for side effects (primarily involving headaches and hangovers). The comedown is often described as having a “dirty” feeling. People often report feeling lethargic, sore, and sweaty.
It’s more common to find 4-FMA as an adulterant in the more popular 4-FA samples — which can be dangerous due to this compound being roughly 4-times more potent. It’s unclear what the toxic dose of 4-FMA is, but it’s common for people to feel pounding headaches and rebound depression lasting several days after taking too much of this drug.
The acute effects of 4-FMA are comparable to MDMA (euphoric and mildly hallucinogenic), but most consider it inferior, and the increased incidences of side effects make it a poor alternative.
4-FMA Specs:
| Chemical Name: | 4-Fluoromethamphetamine |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 4–8 hours |
| Estimated Threshold Dose: | 20 mg mg |
| Common Dose: | 75–125 mg |
| PubChem ID: | 11745017 |
| CAS#: | 351-03-1 |

IUPAC NAME: 1-(4-fluorophenyl)-N-methylpropan-2-amine
MDXX Class
When most people think of “psychedelic amphetamines,” they’re thinking of the methylenedioxyphenethylamine class (shortened to MDXX) — which contains both MDMA and MDA, along with a few others.
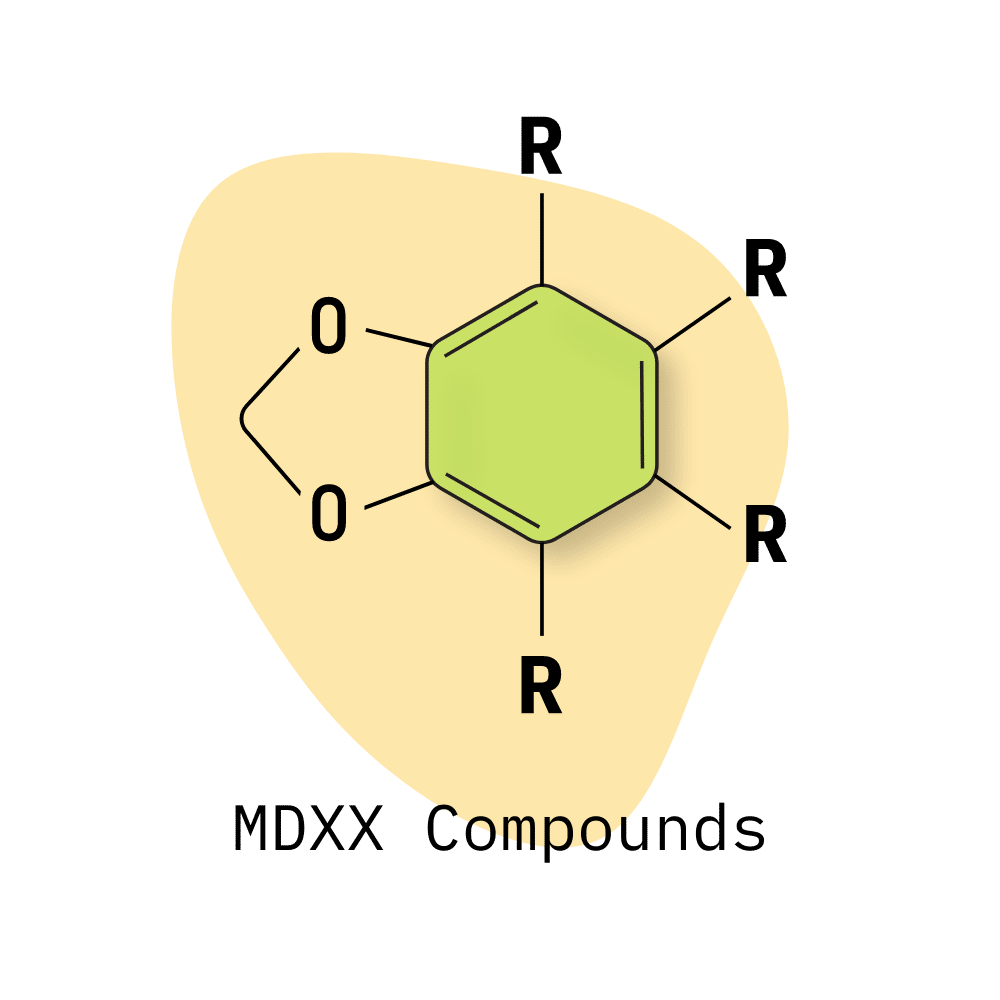
The base compound of the MDXX class is 3,4-methylenedioxyphenethylamine (MDPEA), which is quickly rendered biologically inactive by monoamine oxidase. Additional functional groups can be added to prevent this from happening and provide the characteristic empathogenic, psychedelic, and stimulating effects.
The most important members of this class are MDMA and MDA. There are several other psychoactive compounds in this class, but the majority of them lack psychoactive effects because of how fast MAO deactivates them.
Some people report success with so-called “inactive” MDXX compounds by taking them with an MAO inhibitor. This, in theory, would provide similar synergy as is found in ayahuasca (MAO inhibitor + DMT). Of course, this practice is discouraged as the safety of this is unclear, and there are very good (active) alternatives — namely MDMA — which has a proven track record already.
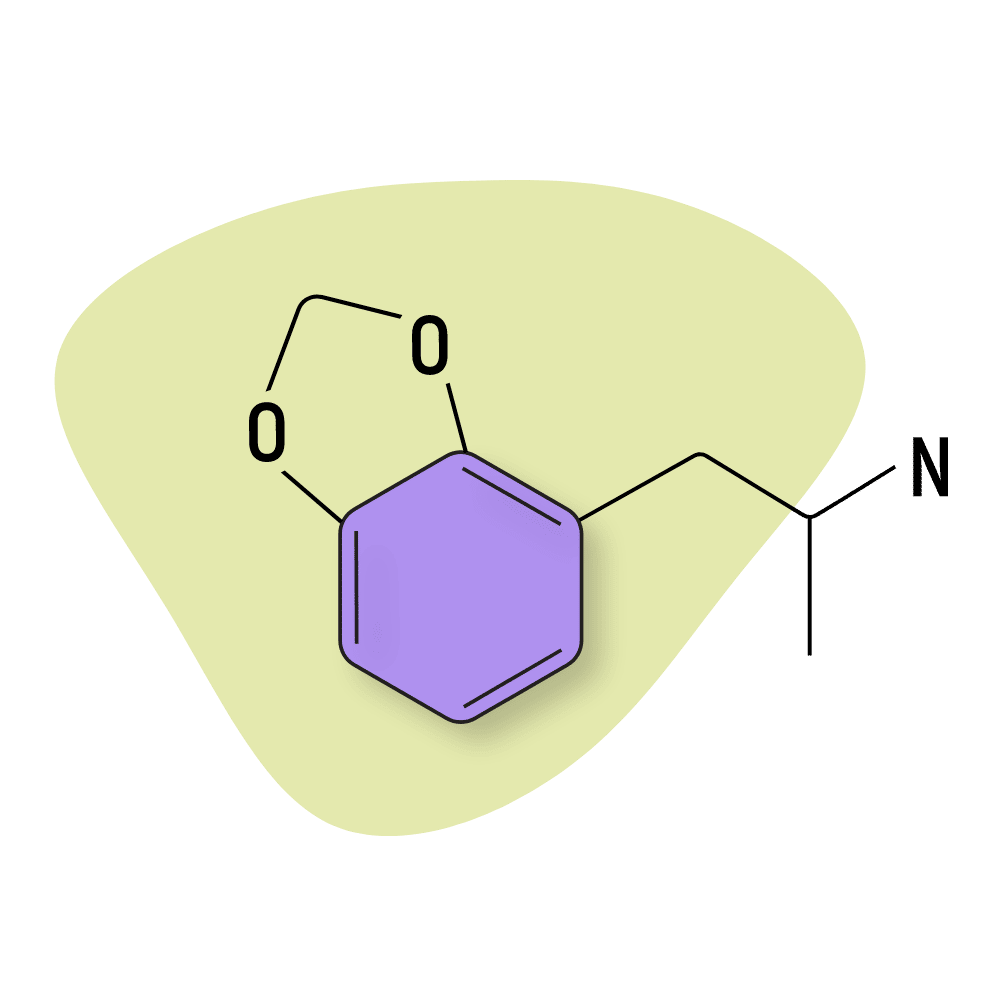
MDA
MDA (3,4-methylenedioxyamphetamine) — sometimes referred to as “Sass” — is closely related to MDMA and is one of the primary metabolites formed as MDMA is broken down by the liver [13].
MDA was invented in 1910 but wasn’t used in medicine until the 1960s where is prescribed as an appetite suppressant under the name Tenamfetamine. It’s no longer used in medicine and is classified as a Schedule I restricted substance or equivalent globally.
This compound is rarely used intentionally as a recreational drug but is a common adulterant in ecstasy tablets. It’s much easier to make than MDMA and produces similar — albeit less desirable — effects than MDMA. MDA is considered weaker, shorter-lasting, and more likely to result in side effects than MDMA. It also has a stronger intoxicating effect than MDMA — rather than feeling more clear-headed, MDA makes users feel a sort of stimulant drunk. Disinhibition is common in higher doses, which can lead users to do things they later regret.
MDA acts as a serotonin-norepinephrine–dopamine releasing agent (SNDRA) to produce its characteristic effects.
MDA Specs:
| Chemical Name: | 3,4-Methylenedioxyamphetamine |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 5–9 hours |
| Estimated Threshold Dose: | 40 mg |
| Common Dose: | 80–150 mg |
| PubChem ID: | 1614 |
| CAS#: | 4764-17-4 |

IUPAC Name: 1-(1,3-benzodioxol-5-yl)propan-2-amine
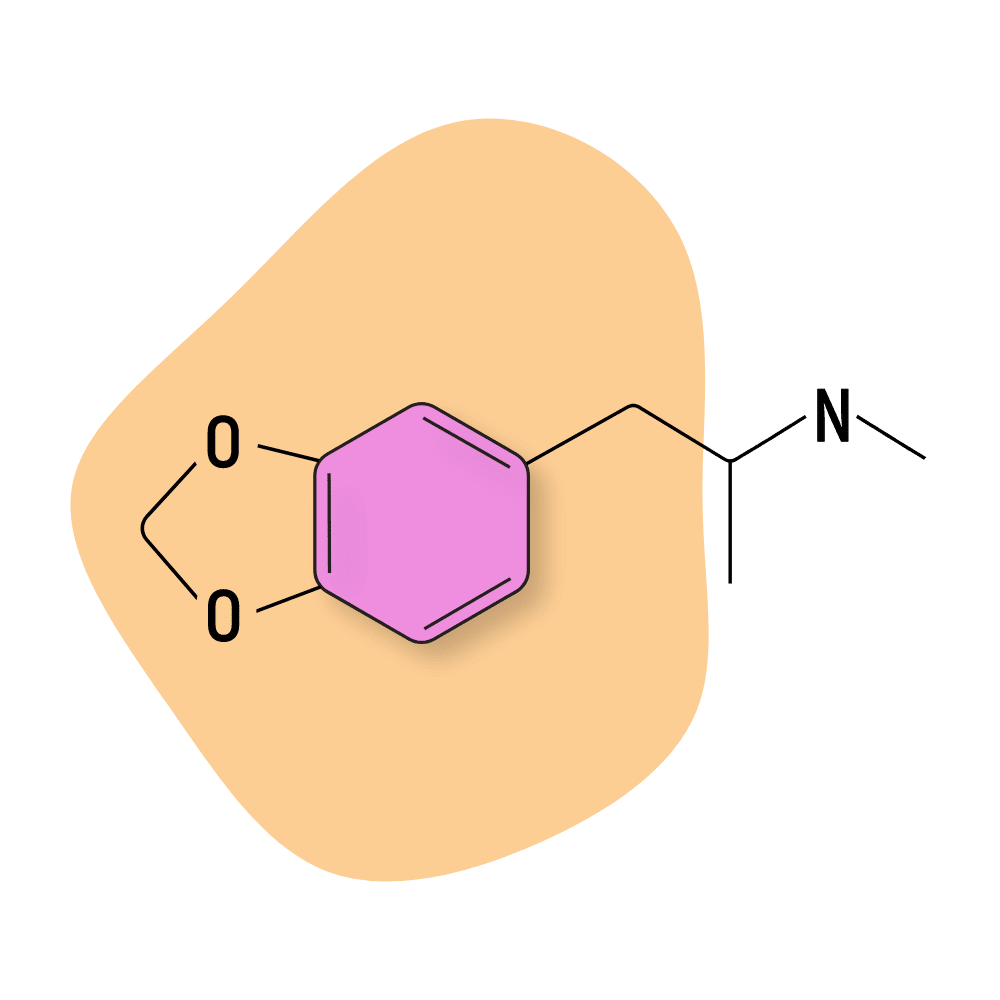
MDMA
MDMA (3,4-Methylenedioxy-N-methylamphetamine) is unique from just about every other compound on this list. Its effects are universally-loved; it’s even being pursued as a medical treatment for conditions such as PTSD and depression and as a tool for couples therapy.
MAPS (the Multidisciplinary Association for Psychedelic Studies) has been leading the charge for legalizing MDMA. The group has been organizing Phase I, II, and III clinical trials for decades and recently submitted a protocol to the FDA to bring MDMA back into clinical therapy.
The effects of MDMA are strongly empathogenic — more so than any other compound on this list. It’s considered a psychedelic because it interacts with the 5HT2A receptors (same target for LSD, psilocybin, and DMT) — albeit to a lesser extent.
MDMA primarily targets the dopamine (D1 and D2) receptors and 5HT2B (serotonin) receptors [10].
MDMA Specs:
| Chemical Name: | 3,4-Methylenedioxy-N-methylamphetamine |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 4–6 hours |
| Estimated Threshold Dose: | 40 mg |
| Common Dose: | 80–150 mg |
| PubChem ID: | 1615 |
| CAS#: | 42542-10-9 |

IUPAC Name: 1-(1,3-benzodioxol-5-yl)-N-methylpropan-2-amine
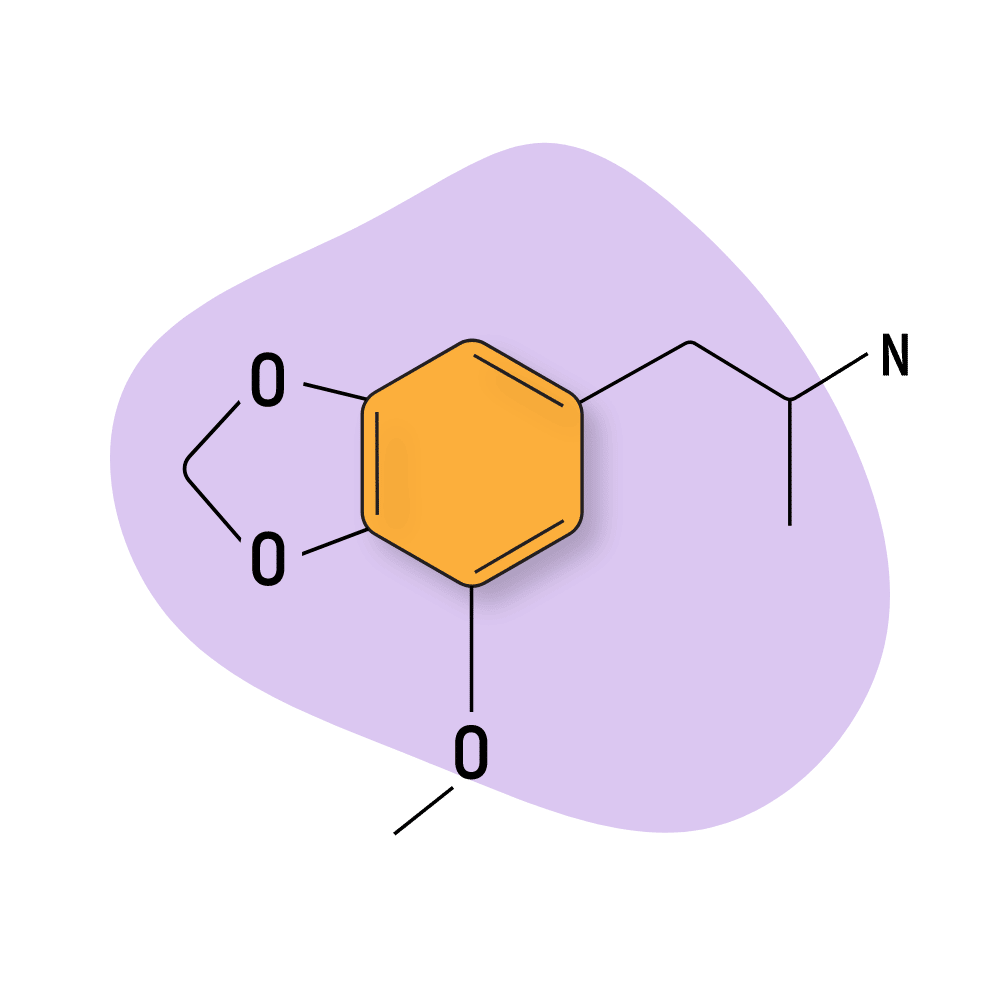
MMDA
MMDA (3,4-Methylenedioxyamphetamine) is a structural analog of MDMA and MDA and shares similar entactogen and stimulating qualities. It’s one of the phenethylamines included in Alexander Shulgin’s book, PiHKAL.
This compound produces feelings of euphoria, mild opened and closed eye visuals, enhanced perception and focus (lower doses), and feelings of openness and connection.
Much like MDMA, many psychologists and researchers believe MMDA may have a legitimate role in psychotherapy. One psychiatrist, Chilean-born Claudio Naranjo, took a deep dive into the therapeutic effects of MMDA in his book The Healing Journey (1973).
MMDA works primarily as a serotonergic compound with only minor activity on the dopaminergic receptors [11,12].
MMDA Specs:
| Chemical Name: | 3,4-Methylenedioxyamphetamine |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 5–7 hours |
| Estimated Threshold Dose: | 60 mg |
| Common Dose: | 100–250 mg |
| PubChem ID: | 26175 |
| CAS#: | 13674-05-0 |

IUPAC Name: 1-(7-methoxy-1,3-benzodioxol-5-yl)propan-2-amine
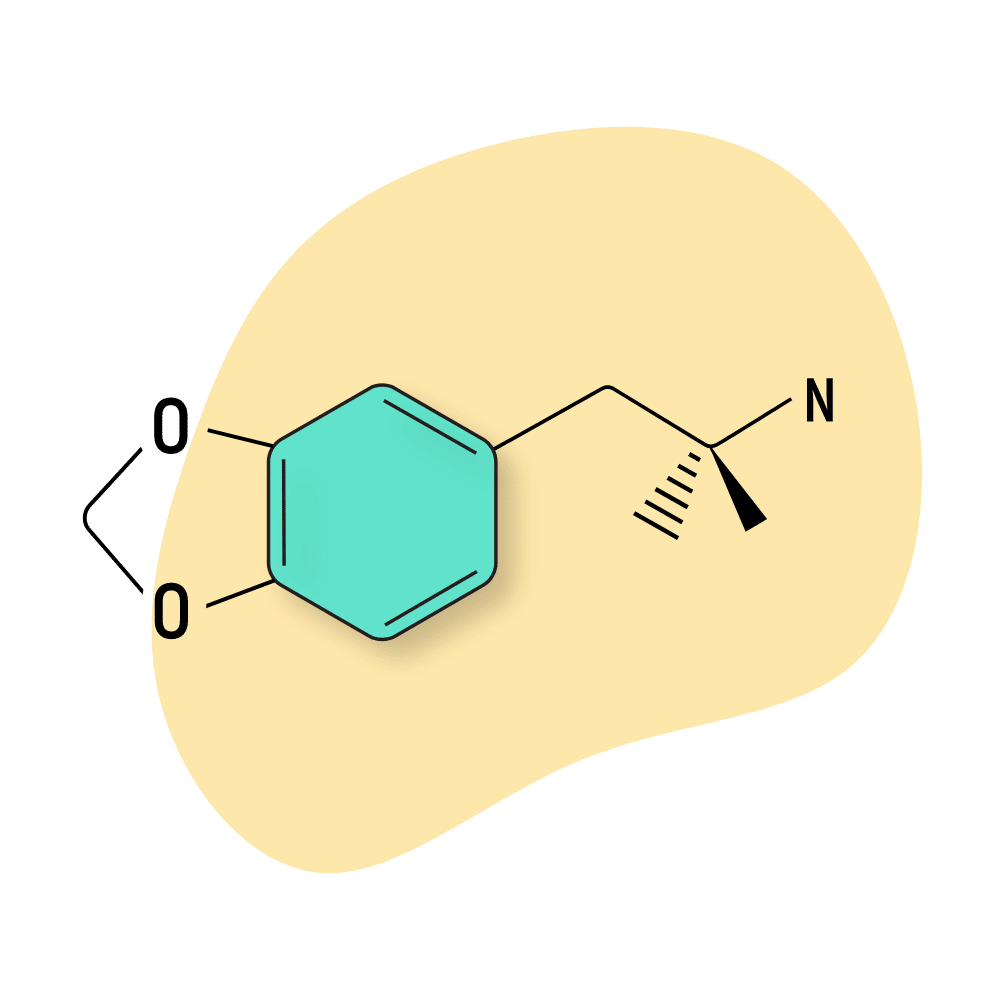
MDPH
MDPH (3,4-Methylenedioxyphentermine) is a lesser-known and milder (but still psychoactive) member of the MDXX class. To date, there are no good studies on this compound to examine how it works and what level of risk it may carry.
Shulgin lists the dosage of this compound as “not as rewarding (stoning) as MDA, and has none of the magic of MDMA.” He reported a foul taste and affinity for making the user feel queasy.
MDPH Specs:
| Chemical Name: | 3,4-Methylenedioxyphentermine |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 3–5 hours |
| Estimated Threshold Dose: | 75 mg |
| Common Dose: | 160–240 mg |
| PubChem ID: | 13020598 |
| CAS#: | 39235-63-7 |

IUPAC NAME: 1-(1,3-benzodioxol-5-yl)-2-methylpropan-2-amine
Other MDXX Amphetamines
There are dozens of other compounds in the MDXX class, but most of them are either entirely inactive or have yet to be tested in any capacity.
No matter how many new amphetamines are created, few offer the same safety profile and desirable effects as the compounds we already have in this class — namely MMDA or MDMA.
Other MDXX Amphetamines Include:
- 2-Methyl-MDA (3,4-Methylenedioxy-2-methylamphetamine)
- 2,3-MDA (2,3-Methylenedioxyamphetamine)
- 5-Me-βk-MDEA (3,4-Methylenedioxy-5-methyl-N-ethylcathinone)
- 5-Methyl-MDA (4,5-Methylenedioxy-3-methylamphetamine)
- 6-Methyl-MDA (4,5-Methylenedioxy-2-methylamphetamine)
- BDB (1,3-Benzodioxolylbutanamine)
- BDP (1,3-Benzodioxolylpentanamine)
- BOH (β-Methoxy-1,3-benzodioxolylbutanamine)
- DMMDA (2,5-Dimethoxy-3,4-methylenedioxyamphetamine)
- DMMDA-2 (4,5-Methylenedioxy-2,3-dimethoxyamphetamine)
- EBDB (N-Ethyl-1,3-benzodioxolylbutanamine)
- EBDP (N-Ethyl-1,3-benzodioxolylpentanamine)
- Lys-MDA (N-(L-lysinamidyl)-3,4-methylenedioxyamphetamine)
- MBDB (N-Methyl-1,3-benzodioxolylbutanamine)
- MBDP (N-Methyl-1,3-benzodioxolylpentanamine)
- MDAL (3,4-Methylenedioxy-N-allylamphetamine)
- MDBU (3,4-Methylenedioxy-N-butylamphetamine)
- MDBZ (3,4-Methylenedioxy-N-benzylamphetamine)
- MDCPM (3,4-Methylenedioxy-N-cyclopropylmethylamphetamine)
- MDDM (3,4-Methylenedioxy-N,N-dimethylamphetamine)
- MDDMPEA (β,N-Dimethyl-3,4-methylenedioxyphenethylamine)
- MDE (3,4-Methylenedioxy-N-ethylamphetamine)
- MMDMA (4,5-Methylenedioxy-3-methoxy-N-methylamphetamine)
- MDEOH (3,4-Methylenedioxy-N-ethyl-N-hydroxyamphetamine)
- MDPEA (3,4-Methylenedioxyphenethylamine)
- MDHOET (3,4-Methylenedioxy-N-(2-hydroxyethyl)amphetamine)
- MDIP (3,4-Methylenedioxy-N-isopropylamphetamine)
- MDMEO (3,4-Methylenedioxy-N-methoxyamphetamine)
- MDMEOET (3,4-Methylenedioxy-N-(2-methoxyethyl)amphetamine)
- MDMOH (3,4-Methylenedioxy-N-methyl-N-hydroxyamphetamine)
- MDMP (3,4-Methylenedioxy-N-methylphentermine)
- MDMPEA (3,4-Methylenedioxy-N-methylphenethylamine)
- MDOH (3,4-Methylenedioxy-N-hydroxyamphetamine)
- MDPEA (3,4-Methylenedioxyphenethylamine)
- MDPH (3,4-Methylenedioxyphentermine)
- MDPL (3,4-Methylenedioxy-N-propargylamphetamine)
- MDPR (3,4-Methylenedioxy-N-propylamphetamine)
- MMDMPEA (4,5-Methylendioxy-3-methoxy-phenethylamine)
- NBOMe-MDPEA (3,4-Methylenedioxy-N-(2-methoxybenzyl)phenethylamine)
- α-cPr-MDMPEA (2-(1,3-Benzodioxol-5-yl)-1-cyclopropyl-N-methylethanamine)
- α-iPr-MDMPEA (2-(1,3-Benzodioxol-5-yl)-1-(propan-2-yl)-N-methylethanamine)
- α-Ph-MDMPEA (2-(1,3-benzodioxol-5-yl)-N-methyl-1-phenylethanamine)
- βk-DMBDB (β-Keto-N,N-dimethyl-1,3-benzodioxolylbutanamine)
- βk-EBDB (β-Keto-N-ethyl-1,3-benzodioxolylbutanamine)
- βk-EBDP (β-Keto-N-ethyl-1,3-benzodioxolylpentanamine)
- βk-MBDB (β-Keto-N-methyl-1,3-benzodioxolylbutanamine)
- βk-MBDP (β-Keto-N-methyl-1,3-benzodioxolylpentanamine)
- βk-MDA (3,4-Methylenedioxycathinone)
- βk-MDEA (3,4-Methylenedioxy-N-ethylcathinone)
- βk-MDMA (3,4-Methylenedioxy-N-methylcathinone)
Substituted Cathinones (AKA Bath Salts)
Substituted cathinones are based on the chemical structure of cathinone — a naturally-occurring alkaloid from the khat plant (Catha edulis). The most common members of this class are MDPV, mephedrone, methylene, and a-PVP (flakka) — but there are over a hundred others as well.
Cathinones are very strong stimulants. Only a small dose is needed to produce effects comparable to basic amphetamines like Adderall or methamphetamine. These compounds are often sold as an alternative to crystal meth or MDMA, but the effects are much more comparable to cocaine.
The basic cathinone structure consists of an amphetamine molecule with a ketone group attached to the beta carbon.
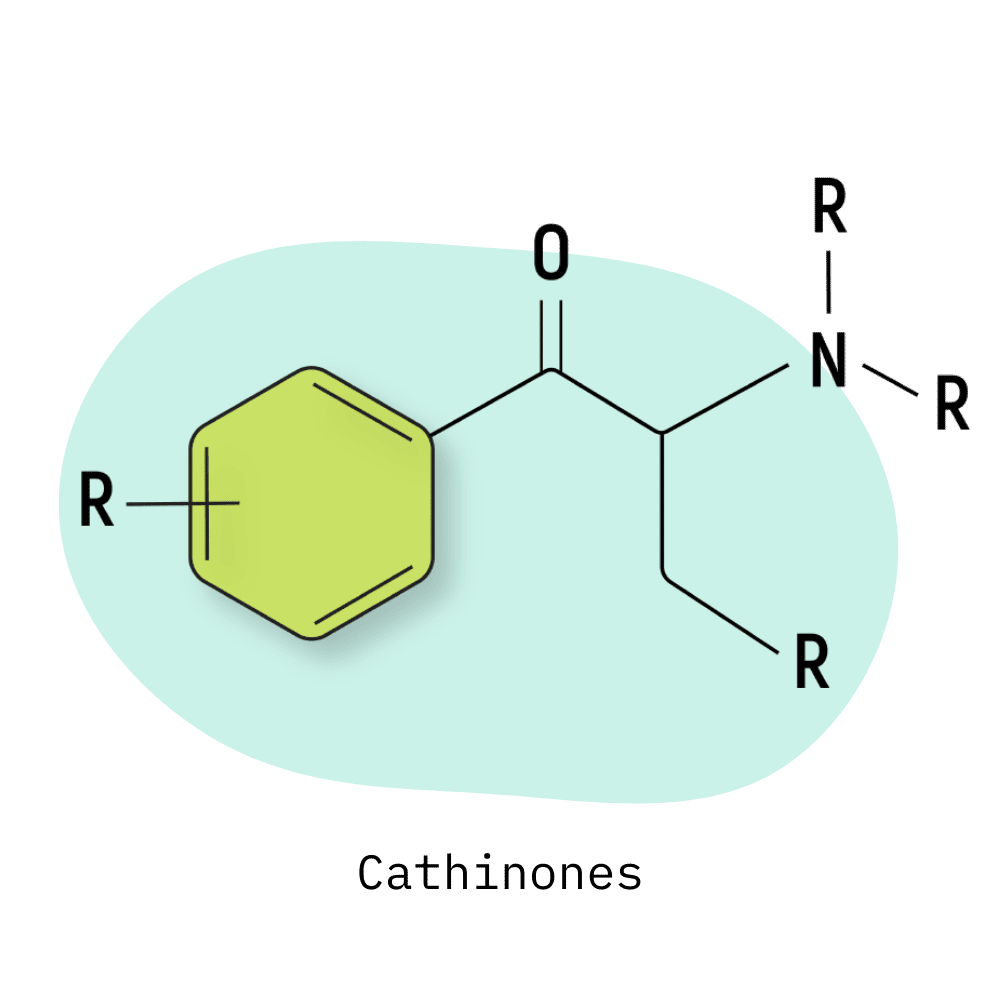
Cathinones work by altering the function of neuronal transporters, including dopamine, norepinephrine, and 5-HT [1]. Some cathinones (such as mephedrone and other ring-substituted analogs) cause a reversal of the transporter; others (such as MDPV and other pyrrolidinophenones) block neurotransmitter reuptake and clearance [2].
The powerful stimulation produced by high doses of synthetic cathinones can result in excited delirium, negative thought loops, panic attacks, hyperthermia, and damage to the neurons. Synthetic cathinones can be fatal in doses not much higher than the conventional psychoactive dose.
Cathinones are well-known to be highly addictive [3].
Here are some of the most common synthetic cathinones and their effects:
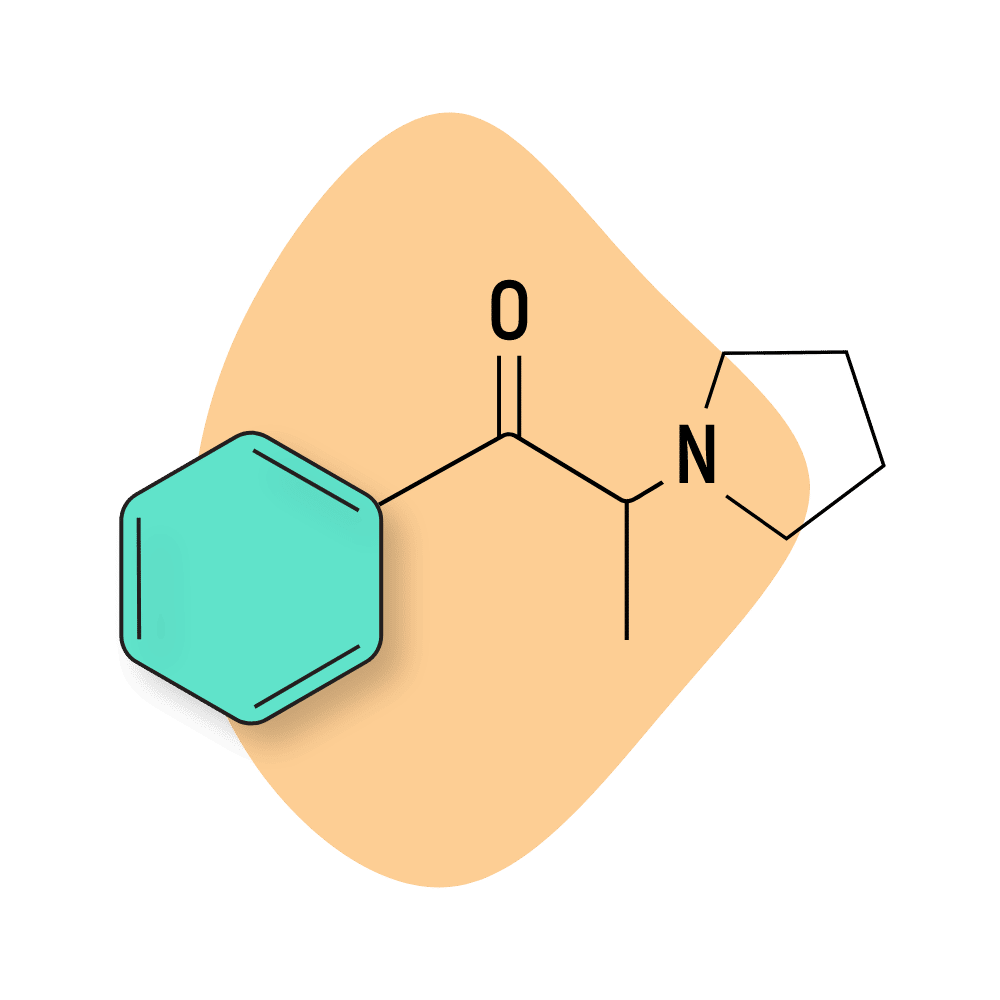
α-PPP
α-PPP (α-Pyrrolidinopropiophenone) is a pyrrolidinophenone derivative structurally similar to a prescription drug called Amfepramone (diethylpropion) which is sold as an appetite suppressant. Pyrrolidinophenones compounds are differentiated by a pyrrolidine ring in an amino group.
This compound is more popular than other pyrrolidinophenone-based cathinones like 4-MePPP but not as popular as α-PVP or MDPV.
α-PPP is sometimes found in cathinone blends (such as NRG-3). It’s been shown to have direct neurotoxic effects in animal studies [4].
All substituted pyrrolidinophenones, including α-PPP, are potent dopamine transporter inhibitors. α-PPP also acts as a partial releasing agent at the norepinephrine transporter [5].
α-PPP Specs:
| Chemical Name | 4′-Methyl-α-pyrrolidinopropiophenone |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 1–3 hours |
| Estimated Threshold Dose (Oral): | 1 mg |
| Common Dose (Oral): | 5–15 mg |
| PubChem ID: | 209045 |
| CAS#: | 19134-50-0 |

IUPAC Name: 1-phenyl-2-pyrrolidin-1-ylpropan-1-one
α-PVP
α-PVP (α-pyrrolidinopentiophenone) is sometimes sold under other names such as Flakka, O-2387, β-keto-prolintane, prolintanone, or desmethylpyrovalerone.
This drug works primarily as a norepinephrine-dopamine reuptake inhibitor.
This powerful stimulant was first invented in 1963 by the German pharmaceutical company Boehringer Ingelheim.
Flakka sat silently on the shelf for several decades until suddenly, around 2014, it began popping up all over the streets in Florida and other parts of the United States. It’s a cheap alternative to other stimulant drugs like cocaine or methamphetamine. You only need about 5–10 mg for effects comparable to about 50 of cocaine (insufflated). It’s roughly ten times as potent as cocaine and about five times more potent than methamphetamine.
The potency of α-PVP is comparable to MDPV.
α-PVP Specs:
| Chemical Name | α-pyrrolidinopentiophenone |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 1–3 hours |
| Estimated Threshold Dose (Oral): | 1 mg |
| Common Dose (Oral): | 5–15 mg |
| PubChem ID: | 11148955 |
| CAS#: | 14530-33-7 |

IUPAC Name: 1-phenyl-2-pyrrolidin-1-ylpentan-1-one
3-MMC (3-Methylmethcathinone)
3-MMC (AKA metaphedrone) is closely related to mephedrone (4-MMC).
Studies suggest 3-MMC is primarily dopaminergic [17] — However, anecdotal reports suggest its effects are more closely aligned with serotonergic stimulants like 2-FEA or MDMA, but with more stimulation. One user suggested that 3-MMC was like mixing MDMA and cocaine.
A lot of people like this amphetamine for its classically stimulating effects mixed with a smooth comedown and euphoric nature.
Overdoses on this drug appear to be rare.
3-MMC Specs:
| Chemical Name | 3-Methylmethcathinone |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 4–7 hours |
| Estimated Threshold Dose: | 20 mg |
| Common Dose: | 75–150 mg |
| PubChem ID: | 71741532 |
| CAS#: | 1246816-62-5 |

IUPAC Name: 2-(methylamino)-1-(3-methylphenyl)propan-1-one
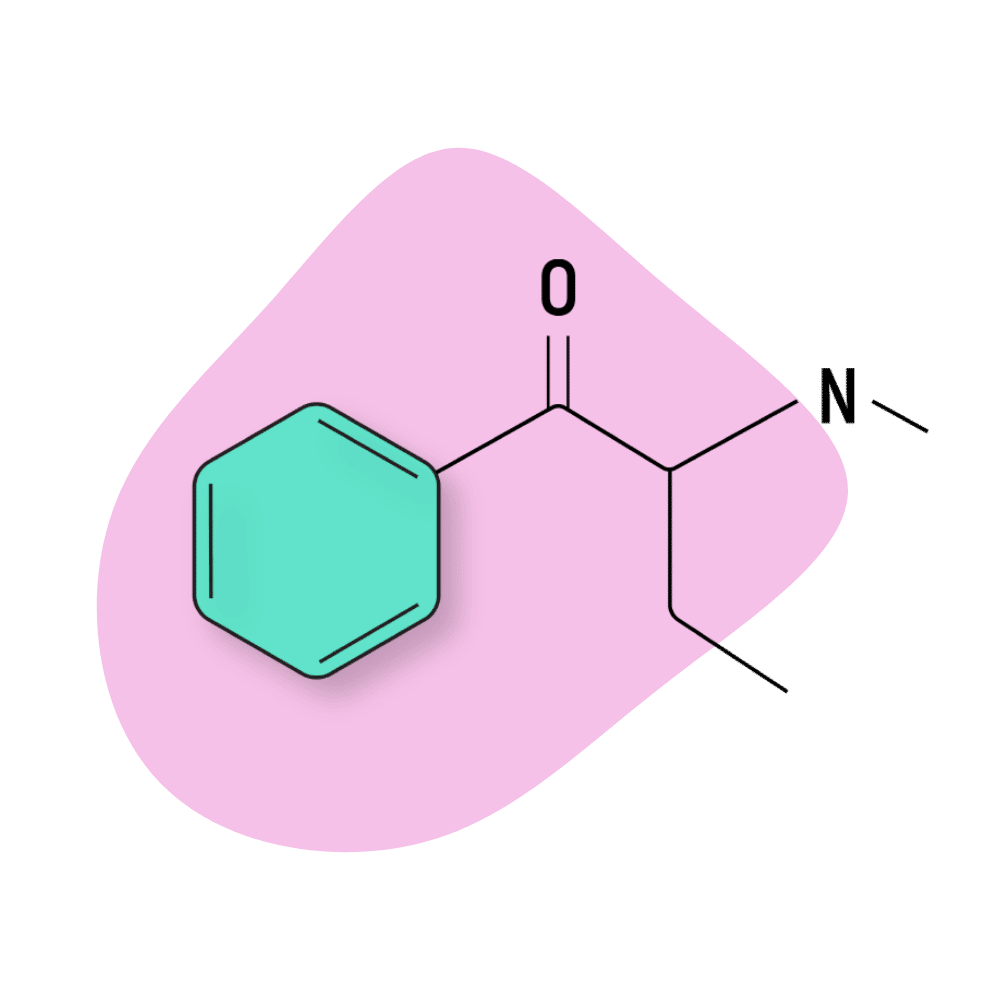
Buphedrone
Buphedrone (α-methylamino-butyrophenone) — AKA MABP — is one of the oldest members in the cathinone chemical family. It was first reported back in 1928 in a report published by researchers at the University of Illinois [6].
This compound is structurally similar to cathinone and cathine but features a β-ketone substituent at the beta carbon and an ethyl group replacing the methyl group.
Buphedrone has been shown to increase the D1 dopamine receptor expression in mice brains — producing comparable effects to amphetamine and MDMA [7]. However, in practice, most users suggest buphedrone is weaker than MDMA and other common amphetamines. Higher doses tend to produce unwanted side effects before they reach a level of intensity comparable to MDMA.
As a result, this amphetamine is rarely used for recreational purposes. There are many other, more desirable compounds available instead.
Buphedrone Specs:
| Chemical Name | α-methylamino-butyrophenone |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 2–5 hours |
| Estimated Threshold Dose: | 10 mg |
| Common Dose: | 30–50 mg |
| PubChem ID: | 53249194 |
| CAS#: | 408332-79-6 |

IUPAC Name: 2-(methylamino)-1-phenylbutan-1-one
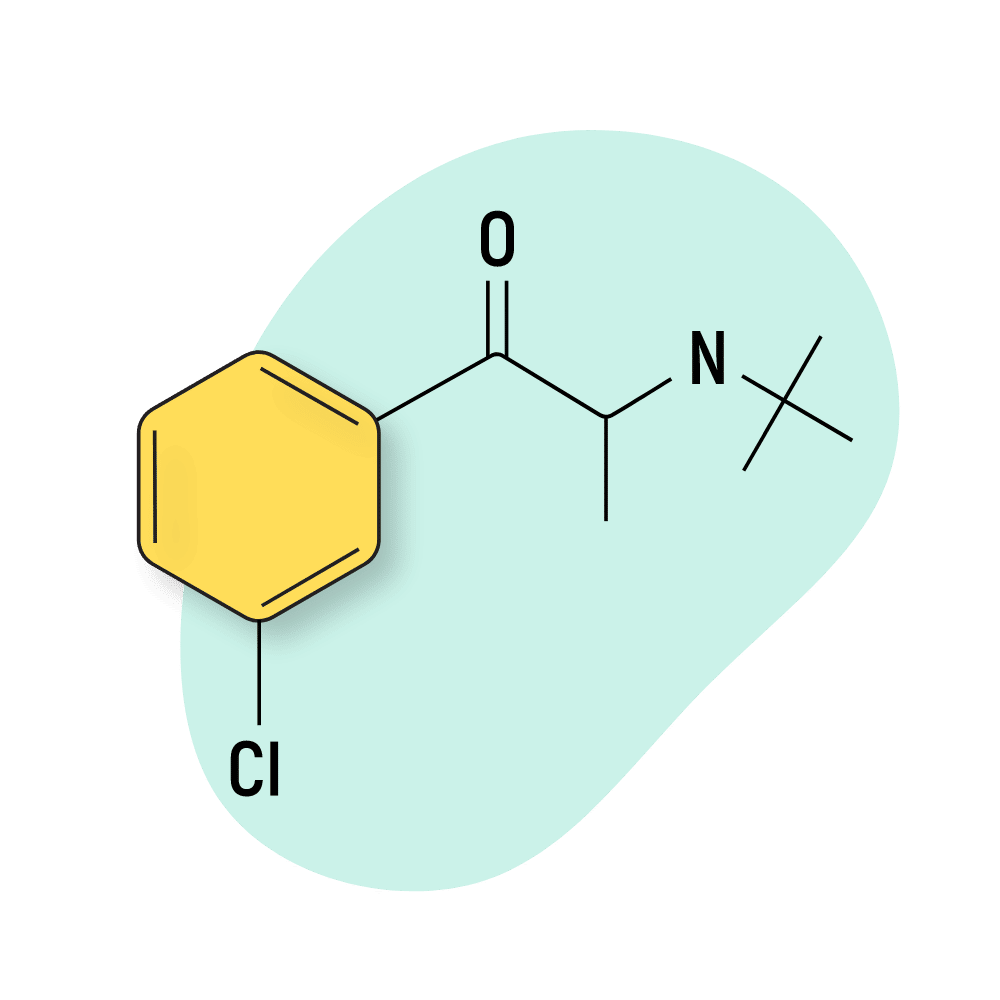
Bupropion (Zyban)
Bupropion is technically classified as a substituted cathinone, but the effects are much different from other members of this family. This drug is further grouped under the aminoketone class and is sold as a prescription antidepressant drug under the trade names Wellbutrin and Zyban (among others). It’s also used as a prescription treatment for smoking cessation.
Many doctors prescribe this medication over other antidepressants because it tends to produce fewer side effects involving sexual dysfunction, weight gain, and fatigue (it’s even used to treat some of these conditions off-label). However, bupropion does carry an increased risk of causing seizures, psychosis, liver toxicity, and overdose.
Bupropion acts as a norepinephrine–dopamine reuptake inhibitor and a nicotinic receptor antagonist [8].
This drug was first invented in 1969 by Nariman Mehta while he was working at Burroughs Wellcome, but it wasn’t approved as a treatment by the FDA until much later, in 1985. It’s currently listed as an essential medicine by the World Health Organization (WHO).
Bupropion Specs:
| Chemical Name | Bupropion |
| Status: | Approved |
| Duration of Effects: | 2–3 hours |
| Estimated Threshold Dose: | 75 mg |
| Common Dose: | 150–450 mg |
| PubChem ID: | 62884 |
| CAS#: | 31677-93-7 |

IUPAC Name: 2-(tert-butylamino)-1-(3-chlorophenyl)propan-1-one;hydrochloride
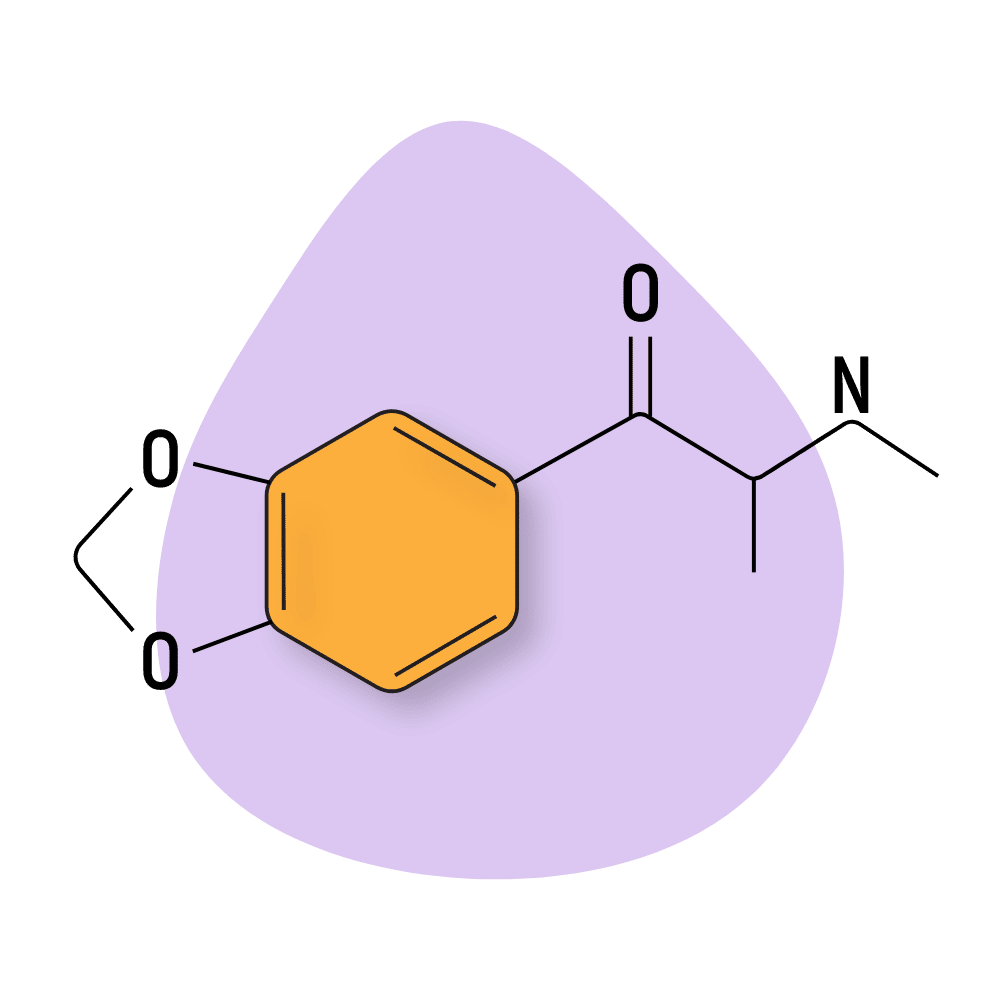
Butylone
Butylone (β-keto-N-methylbenzodioxolylbutanamine) is a next-generation synthetic cathinone with powerful stimulating activity. It’s the β-keto analog of MDBD and is sometimes referred to as βk-MBDB or B1.
This compound is a common adulterant in MDMA pills, but the effect profile is much different — it’s much more psychostimulant than entactogen, whereas MDMA is more entactogen than psychostimulant.
Butylone is considered slightly weaker than methylene and ethylene — which are the closest in terms of chemical structure.
Butylone Specs:
| Chemical Name | β-keto-N-methylbenzodioxolylbutanamine |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 3–5 hours |
| Estimated Threshold Dose: | 20 mg |
| Common Dose: | 70–100 mg |
| PubChem ID: | 56843046 |
| CAS#: | 802575-11-7 |

IUPAC Name: 1-(1,3-benzodioxol-5-yl)-2-(methylamino)butan-1-one
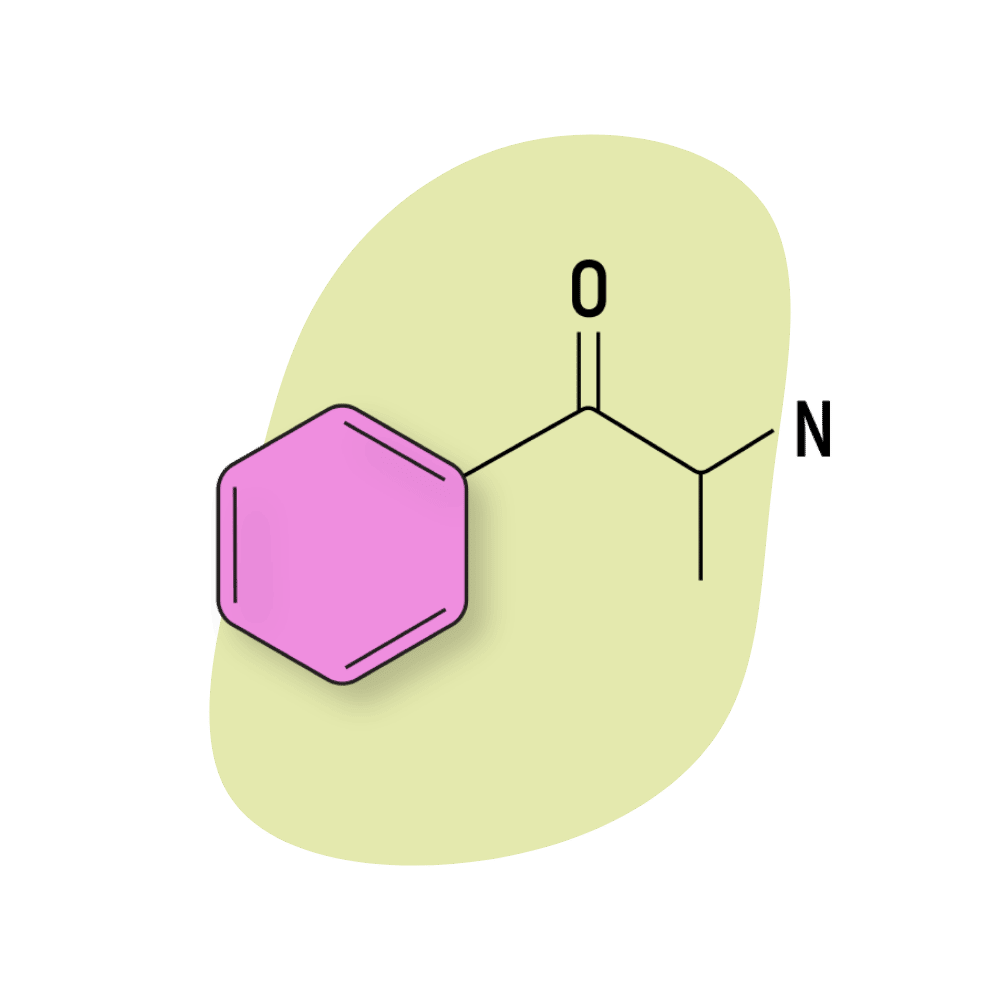
Cathinone
Cathinone (sometimes referred to as benzoylethanamine or β-keto-amphetamine) is the basic starting structure for all substituted cathinones. It’s a naturally-occurring monoamine alkaloid in the Catha edulis (khat) plant responsible for much of the herb’s psychostimulant effect profile.
Cathinone can be extracted from the khat plant but is more commonly created synthetically from propiophenone. The only structural difference between cathinone and amphetamine is the presence of a ketone group on the cathinone molecule.
Like other monoamine alkaloids, cathinone works by stimulating the release of dopamine and blocking the reuptake of norepinephrine and serotonin [9].
Cathinone Specs:
| Chemical Name | Cathinone |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 3–5 hours |
| Estimated Threshold Dose: | 5 mg |
| Common Dose: | 10–20 mg |
| PubChem ID: | 62258 |
| CAS#: | 71031-15-7 |

IUPAC Name: (2S)-2-amino-1-phenylpropan-1-one
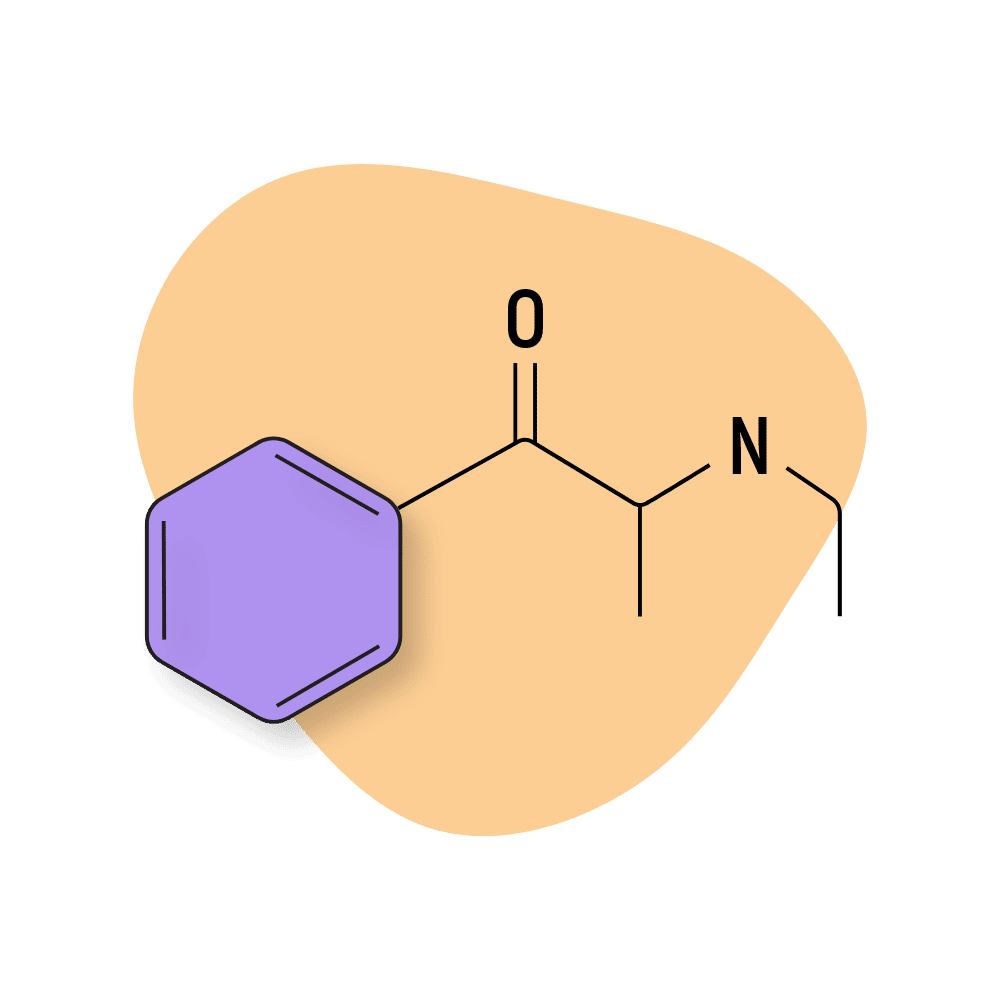
Ethcathinone (ETH-CAT)
Ethcathinone (ETH-CAT) was first reported in a 2006 paper by Richard Rothman and Michael Baumann. Several years later, it was sold as a legal high but promptly banned by the US and the European Union.
ETH-CAT remains popular today for its generally milder (but still very strong) stimulating effect profile and long-lasting effects. The effects peak quickly before tapering into a more Adderall-like high lasting several hours.
This compound is most structurally related to MCAT and cathinone.
ETH-CAT Specs:
| Chemical Name | Ethcathinone |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 4–7 hours |
| Estimated Threshold Dose: | 60 mg |
| Common Dose: | 150–225 mg |
| PubChem ID: | 458519 |
| CAS#: | 18259-37-5 |

IUPAC Name: 2-(ethylamino)-1-phenylpropan-1-one
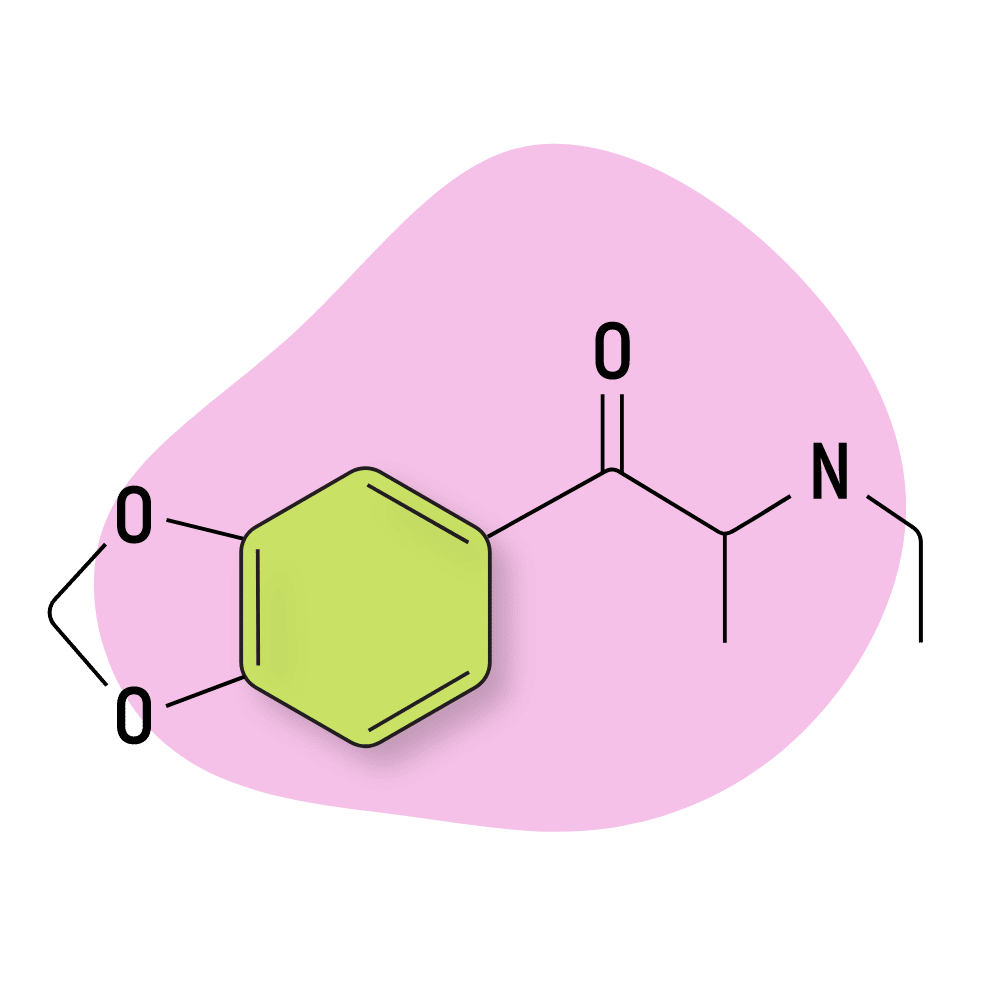
Ethylone
Ethylone (3,4-methylenedioxy-N-ethylcathinone) — sometimes referred to as MDEC or βk-MDEA — is the β-keto analog of MDEA (an MDXX amphetamine).
Despite how similar the structure of ethylone is to MDMA and MDEA, the effects are distinctly different. The effects of ethylone, like most other cathinones, are more comparable to cocaine than to MDMA or MDA. This compound is highly stimulating and has little if any, entactogenic activity.
Ethylone is a common adulterant of other synthetic cathinones, particularly methylone and ecstasy.
Ethylone acts as a serotonin, norepinephrine, and dopamine reuptake inhibitor [14].
Ethylone Specs:
| Chemical Name | 3,4-methylenedioxy-N-ethylcathinone |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 2–4 hours |
| Estimated Threshold Dose: | 75 mg |
| Common Dose: | 200–250 mg |
| PubChem ID: | 57252245 |
| CAS#: | 1112937-64-0 |

IUPAC Name: 1-(1,3-benzodioxol-5-yl)-2-(ethylamino)propan-1-one
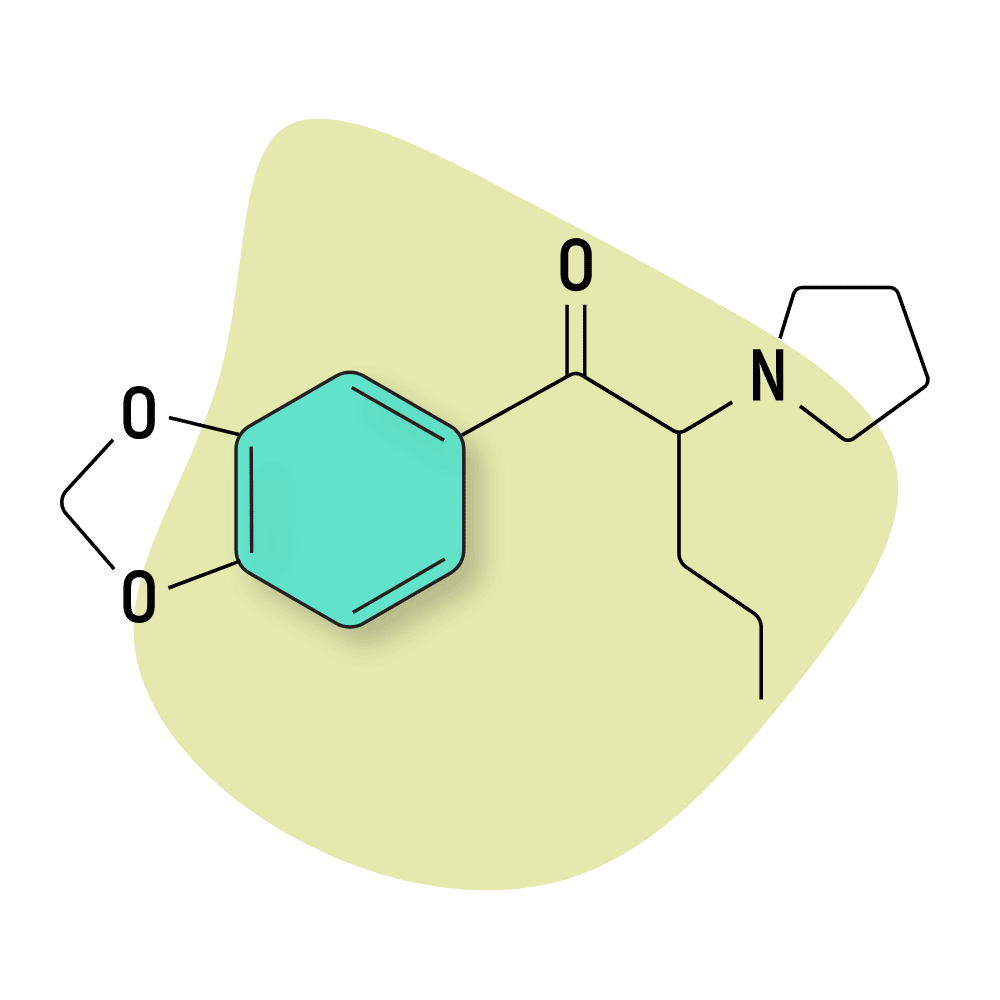
MDPV
MDPV (methylenedioxypyrovalerone) is one of the three first-generation substituted cathinones (along with methylone and mephedrone). It was first developed in the 1960s by Boehringer Ingelheim but didn’t become popular until the early 2000s after it entered the designer drug market.
This drug is extremely potent — it’s roughly 6-times more active on the dopamine receptor than the norepinephrine receptor [2]. The threshold dose is just 2 mg, and the standard psychoactive dose is somewhere between 8 and 14 mg.
This drug has a very narrow dosage window — taking just a little bit too much can lead to psychosis, heart failure, stroke, or permanent brain damage. Addiction is also very common with this substance.
MDPV is structurally similar to α-PVP. Both compounds work by altering norepinephrine and dopamine functions without having much impact on serotonin.
MDPV Specs:
| Chemical Name | Methylenedioxypyrovalerone |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 3–7 hours |
| Estimated Threshold Dose: | 2 mg |
| Common Dose: | 5–15 mg |
| PubChem ID: | 20111961 |
| CAS#: | 687603-66-3 |

IUPAC Name: 1-(1,3-benzodioxol-5-yl)-2-pyrrolidin-1-ylpentan-1-one
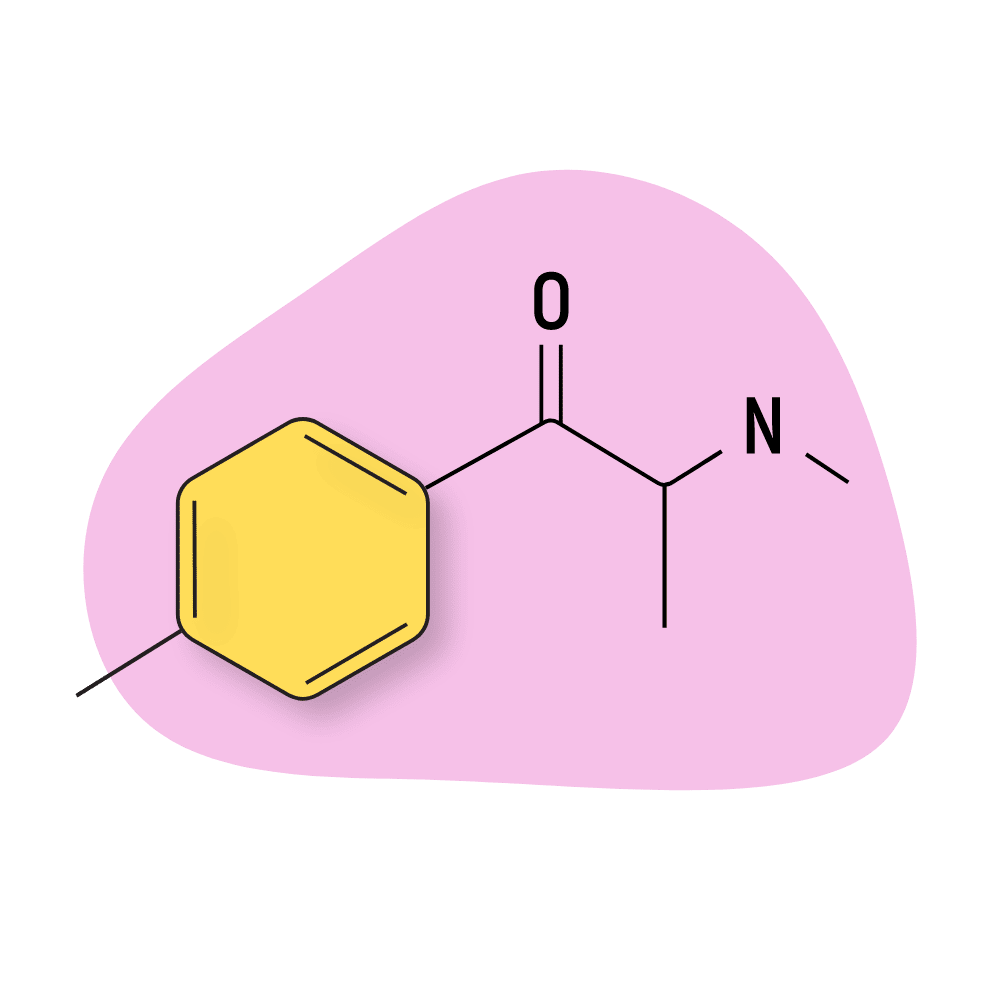
4-MMC (Mephedrone)
Mephedrone (AKA 4-methylmethcathinone and 4-MMC) is a first-generation synthetic cathinone that’s been on the designer drug market since at least 1999. It’s sold under names such as M-CAT, Drone, Meow Meow, White Magic, and others.
The effects of mephedrone are similar to MDMA but with markedly less empathogenic qualities. This drug primarily increases extracellular concentrations of dopamine and serotonin, with only a mild increase in norepinephrine.
This cathinone is roughly half as potent as MDPV and widely considered to be “less” dangerous. However, 4-MMC still carries a significant risk of overdose, psychosis, heart and brain damage, and addiction.
4-MMC (Mephedrone) Specs:
| Chemical Name | 4-methylmethcathinone |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 3–6 hours |
| Estimated Threshold Dose: | 5 mg |
| Common Dose: | 100–200 mg |
| PubChem ID: | 45266826 |
| CAS#: | 1189805-46-6 |

IUPAC Name: 2-(methylamino)-1-(4-methylphenyl)propan-1-one
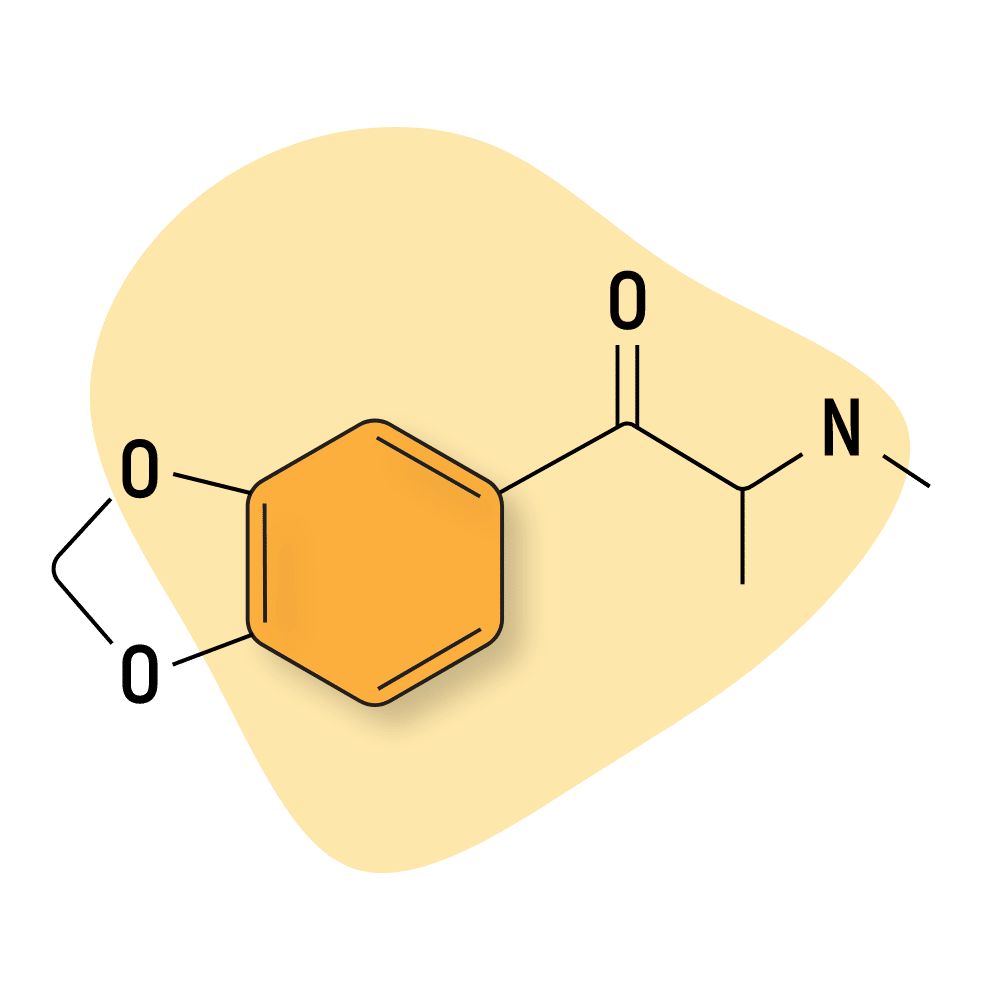
Methylone
Methylone (3,4-methylenedioxy-N-methylcathinone) is often sold as M1, MDMC, or βk-MDMA.
This compound is the substituted cathinone analog of MDMA. Methylone and MDMA are differentiated only by the substitution of 2 hydrogen atoms with oxygen in the β position of the phenethylamine core.
Alexander Shulgin described methylone as “almost the same potency of MDMA, but it does not produce the same effects. It has an almost antidepressant action, pleasant and positive, but not the unique magic of MDMA.”
Methylone Specs:
| Chemical Name | 3,4-Methylenedioxy-N-methcathinone |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 2.5–4 hours |
| Estimated Threshold Dose: | 75 mg |
| Common Dose: | 150–225 mg |
| PubChem ID: | 45789647 |
| CAS#: | 186028-79-5 |

IUPAC NAME: 1-(1,3-benzodioxol-5-yl)-2-(methylamino)propan-1-one
Other Synthetic Cathinones
There are a lot of possible combinations for synthetic cathinones, but few have become as popular as drugs like α-PPP, α-PVP, 3-MMC, 4-MMC, and MDPV.
Most cathinones haven’t been explored enough to provide context for their safe use.
List of other “novel” cathinones:
- α-PBP
- α-PHP
- α-PHpP (PV8)
- α-PPP
- 2-MMC
- 3-CEC
- 3-CMC
- 3-FMC
- 4-CEC
- 4-CMC
- 4-ethylpentedrone
- 4-MC
- 4-MEC
- 4-methyl-NEP
- 4F-α-PVP
- Mexedrone
- N-ethyl-nor-heptedrone
- N-ethyl-nor-hexedrone
- N-ethyl-nor-pentedrone (NEP)
- Pentedrone
DOX Compounds
The DOX family consists of a long list of substituted dimethoxyamphetamines, most of which act as the middle ground between psychedelics and stimulants. Their effects are somewhat similar to the 2CX family — with a unique cartoon-like vibrancy, dramatic boost in energy, and deep psychedelic introspection in higher doses.
This class is characterized by the presence of an amphetamine backbone with two methoxy groups in positions 2 and 5 and a variable functional group in position 4.
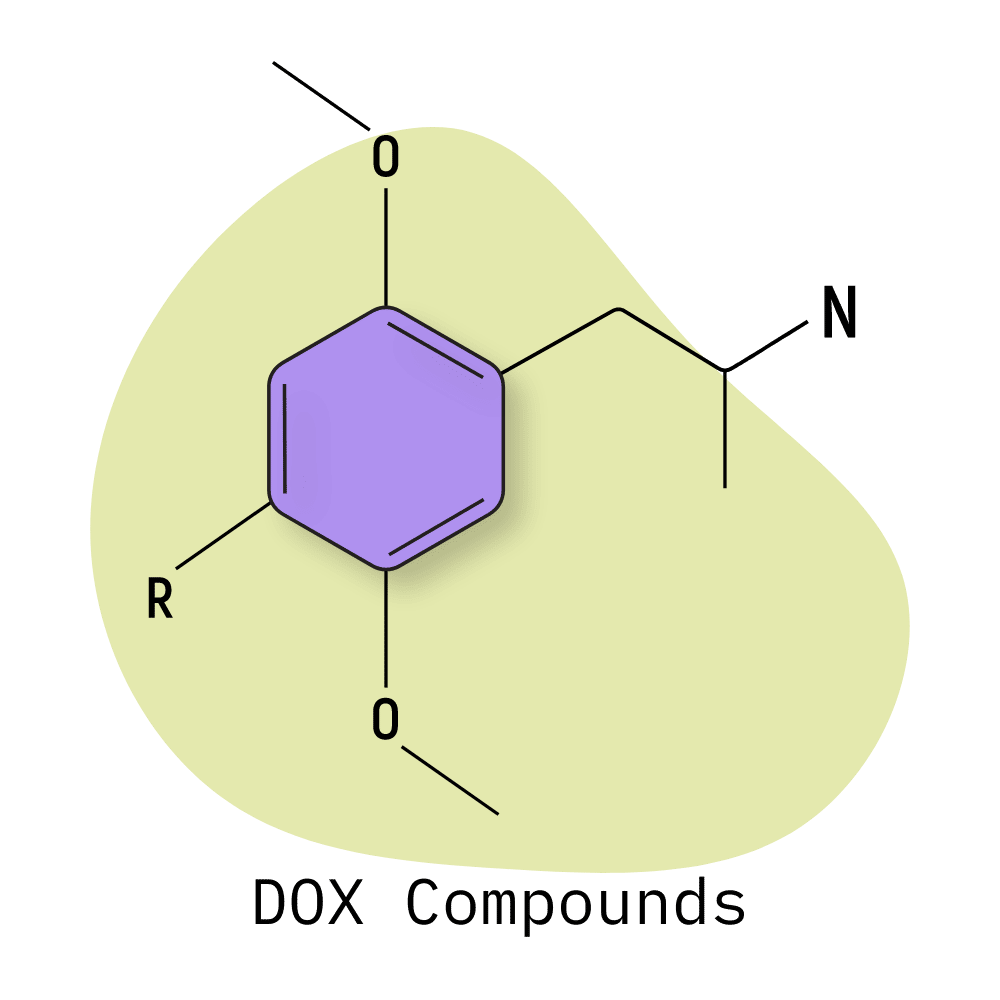
Early members of this class were first identified and synthesized by Alexander Shulgin in the 1970s. Shulgin published his findings, along with the first recorded trip reports for these substances in his book, PiHKAL — along with a variety of other mescaline-inspired compounds (phenethylamines) such as the 2CX family of drugs.
DOX compounds are structurally similar to the 2CX family — the difference being that only the DOX compounds are classified as amphetamines.
Qualitatively, DOX drugs take much longer to kick in (up to 3 hours) but last longer and are substantially more potent than 2CX drugs. It’s easy to take too much, and the effects can vary substantially depending on the dose.
The DOX class of substituted amphetamines is strong enough to deliver psychedelic doses on blotter paper, which has led many unethical clandestine drug manufacturers to sell DOX-coated blotters under the guise of LSD.
The only other classes of drugs potent enough to remain active at sub-milligram doses are the lysergamides, benzodiazepines, and the NBOMes.
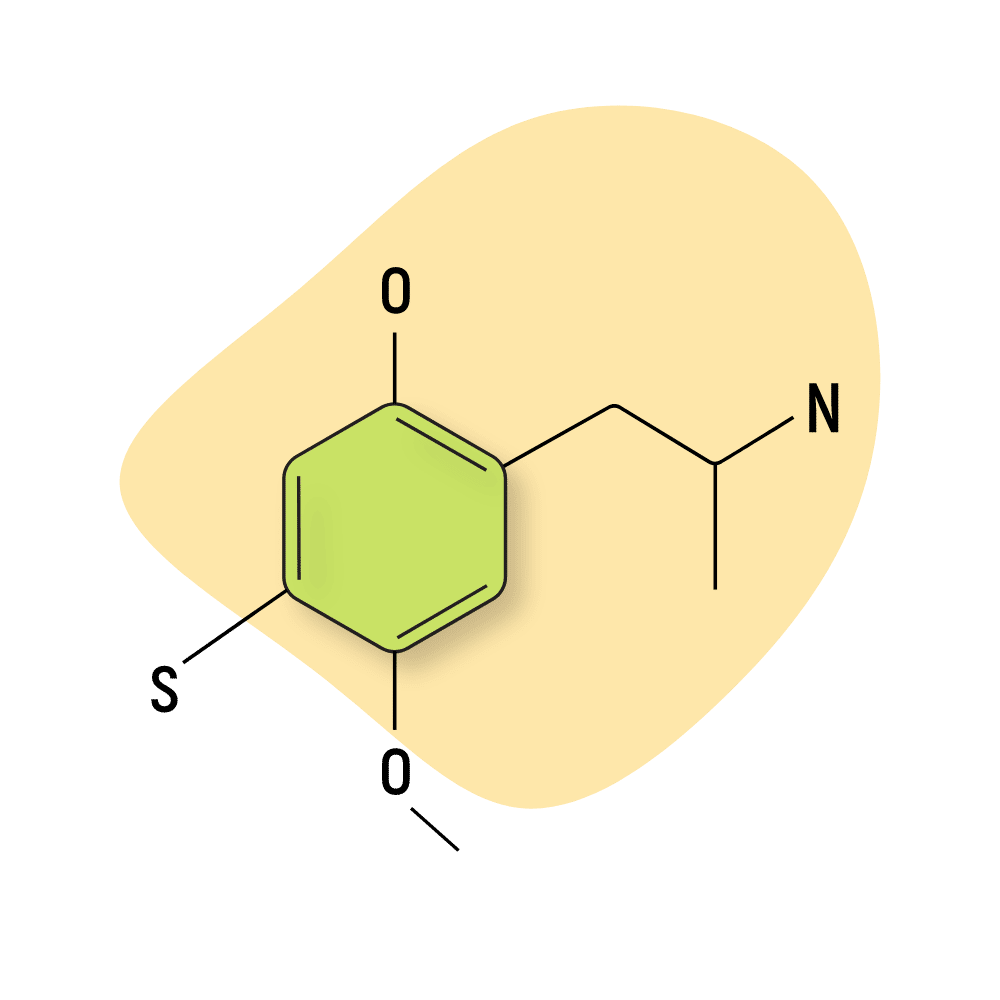
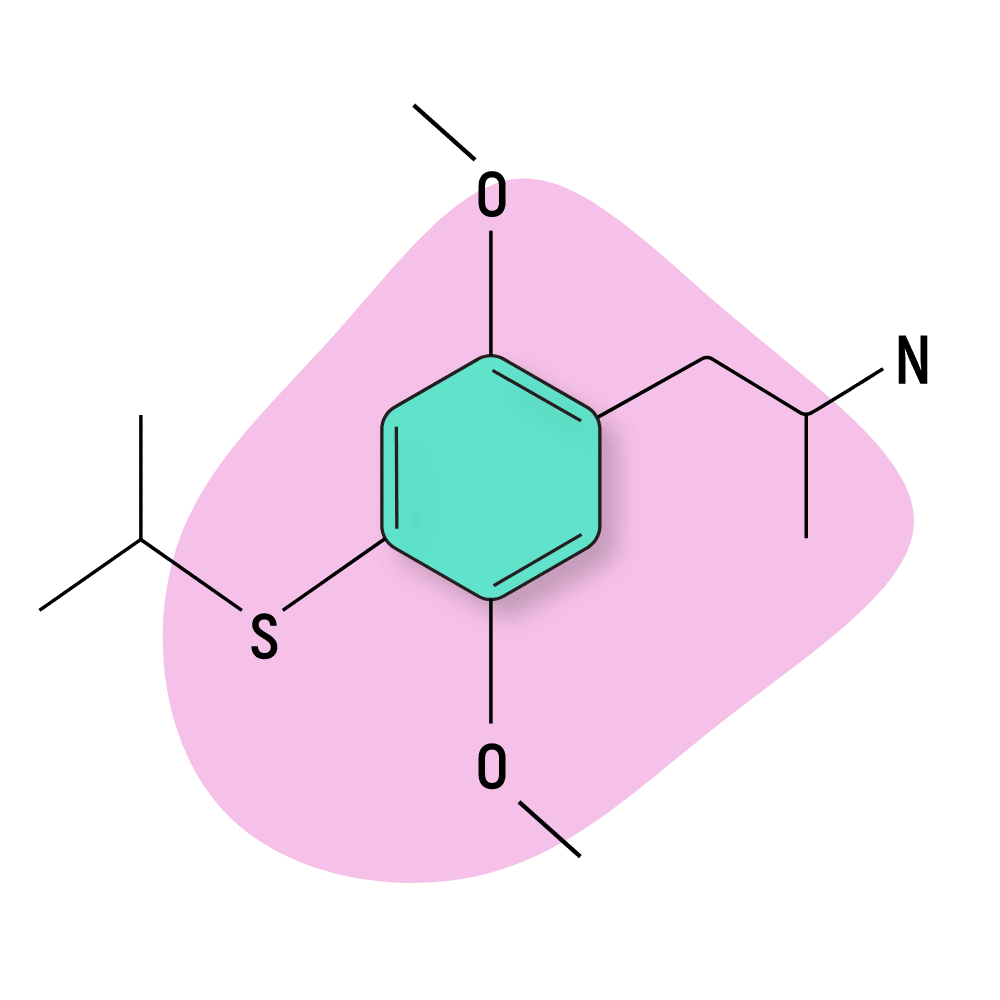
Aleph
Aleph (2,5-dimethoxy-4-methylthioamphetamine) — also known as DOT — was first reported by Alexander Shulgin in the 1990s. This compound is characterized by the addition of a sulfur group on position-4 of the central ring moiety.
Shulgin reported four active homologs of this compound, Aleph-2, Aleph-4, Aleph-6, and Aleph-7.
All four are potent and long-lasting psychostimulants, entactogens, and psychedelics. Aleph-7 is listed as the strongest (according to Shulgin). It’s roughly twice as potent as Aleph-2 and Aleph-4 and nearly four times stronger than Aleph-6.
These drugs’ effects are all similar, producing intellectual stimulation, perceptual changes to audio and visual information processing, and distinct body sensations. As amphetamines, this class produces a characteristic stimulating effect that remains throughout most of the experience.
Aleph compounds are structural analogs of the 2C-T-X group (a sub-group of the greater 2C-X family of psychedelics):
- Aleph-2 — analog of 2C-T-2
- Aleph-4 — analog of 2C-T-4
- Aleph-6 — analog of 2C-T-6
- Aleph-7 — analog of 2C-T-7
The duration of effects for this class ranges from 8 to 30 hours (depending on the dose and individual metabolic factors).
Aleph-2 Specs:
| Chemical Name | 2,5-dimethoxy-4-ethylthioamphetamine |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 8–16 hours |
| Estimated Threshold Dose: | 3 mg |
| Common Dose: | 4–8 mg |
| PubChem ID: | 10264356 |
| CAS#: | 185562-00-9 |

IUPAC Name: 1-(4-ethylsulfanyl-2,5-dimethoxyphenyl)propan-2-amine
Aleph-4 Specs:
| Chemical Name | 2,5-Dimethoxy-4-iso-propylthioamphetamine |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 12–20 hours |
| Estimated Threshold Dose: | 4 mg |
| Common Dose: | 7–12 mg |
| PubChem ID: | 44350301 |
| CAS#: | 123643-26-5 |

IUPAC Name: 1-(2,5-dimethoxy-4-propan-2-ylsulfanylphenyl)propan-2-amine
Aleph-6 Specs:
| Chemical Name | 2,5-Dimethoxy-4-phenylthioamphetamine |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | Unspecified (very long) |
| Estimated Threshold Dose: | 20 mg |
| Common Dose: | >40 mg |
| PubChem ID: | 44719475 |
| CAS#: | 952006-44-9 |

IUPAC Name: 1-(2,5-dimethoxy-4-phenylsulfanylphenyl)propan-2-amine
Aleph-7 Specs:
| Chemical Name | 2,5-Dimethoxy-4-propylthioamphetamine |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 15–30 hours |
| Estimated Threshold Dose: | 3 mg |
| Common Dose: | 4–7 mg |
| PubChem ID: | 11197521 |
| CAS#: | 207740-16-7 |

IUPAC Name: 1-(2,5-dimethoxy-4-propylsulfanylphenyl)propan-2-amine
DOAM
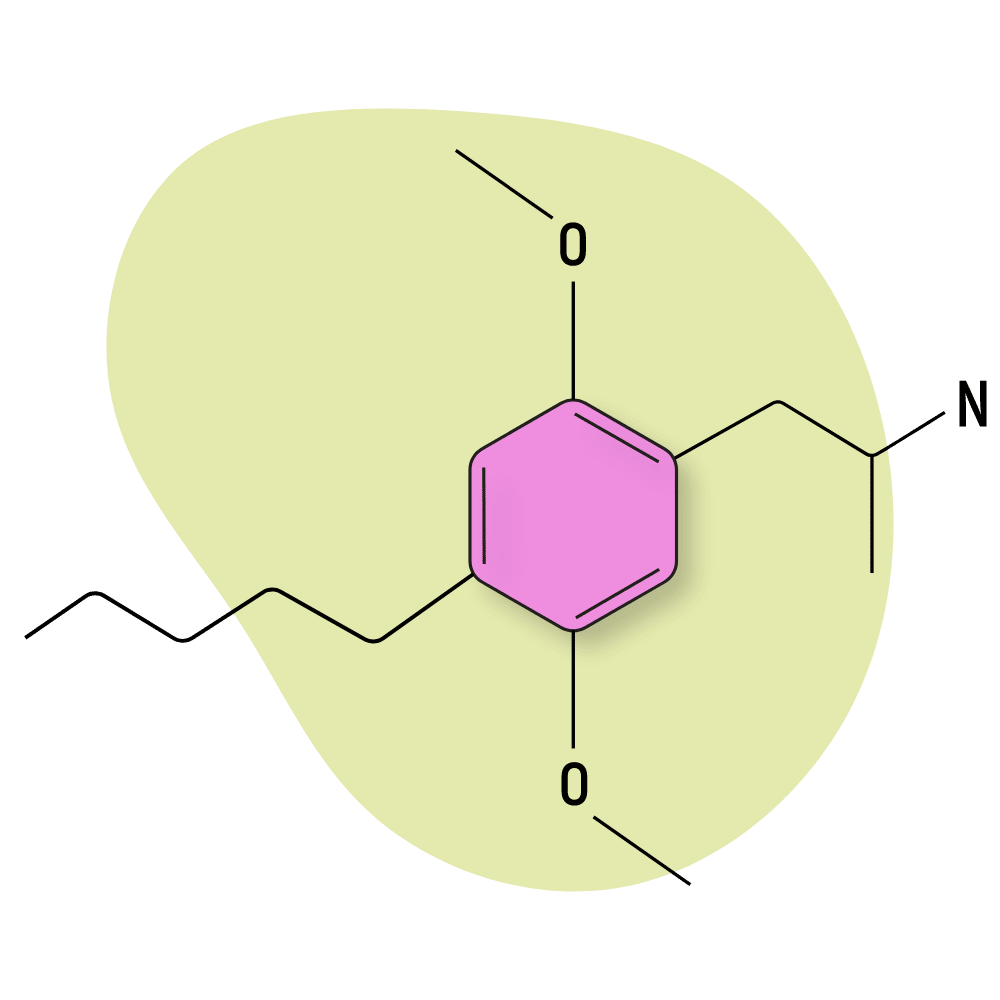
DOAM (2,5-Dimethoxy-4-amylamphetamine) is the 4-alkyl version of Shulgin’s so-called “Ten Classic Ladies” — a class of homologous DOX compounds that include DOM, DOET, DOPR, and DOBU — each one with slight differences in the functional side chains.
Not much is known about the effects of this compound, and it hasn’t been very popular in the research chemical space, but the psychoactivity was reported as being relatively enjoyable by Shulgin at the threshold dose of 10 mg.
DOAM Specs:
| Chemical Name | 2,5-Dimethoxy-4-amylamphetamine |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | Unknown |
| Estimated Threshold Dose: | 5 mg |
| Common Dose: | >10 mg |
| PubChem ID: | 12262512 |
| CAS#: | 63779-90-8 |

IUPAC Name: 1-(2,5-dimethoxy-4-pentylphenyl)propan-2-amine
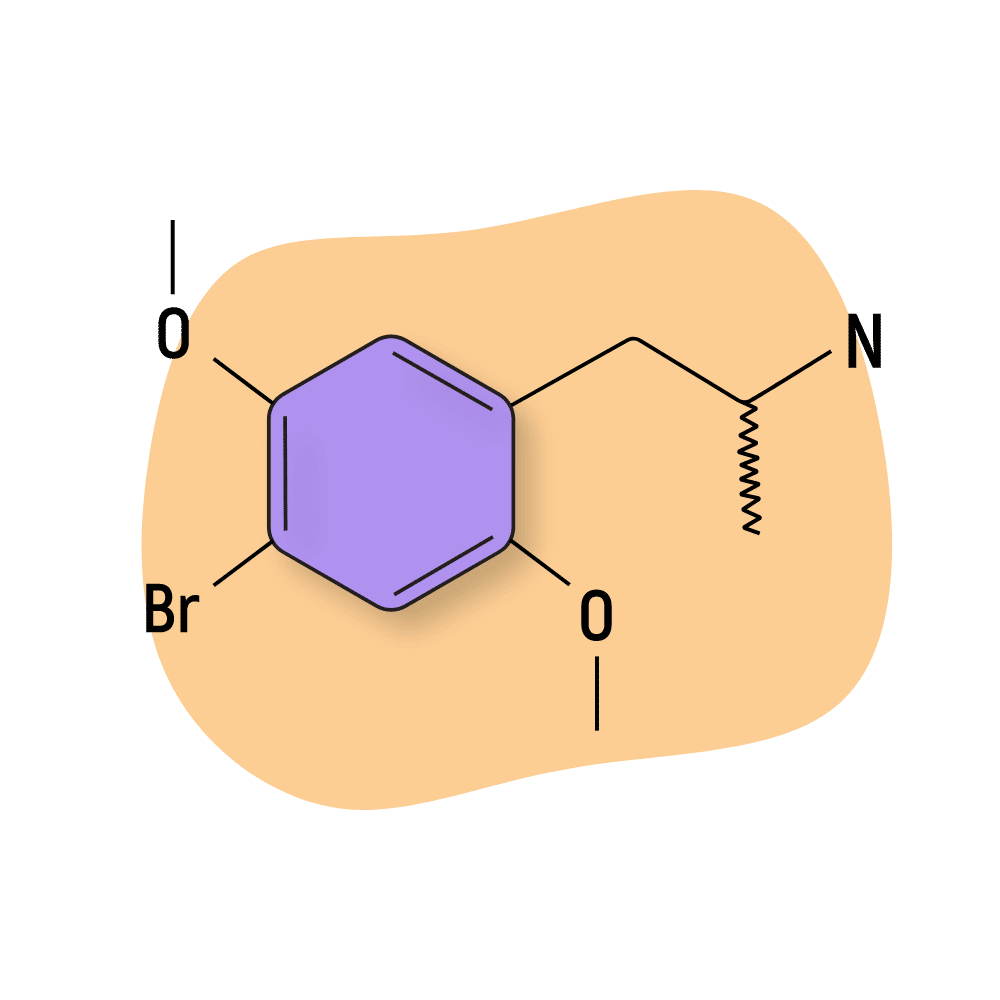
DOB
DOB (4-Bromo-2,5-dimethoxyamphetamine) is the amphetamine analog of the popular 2C psychedelic, 2C-B. It’s sometimes sold under a different name, brolamfetamine.
This compound is best known for its exceptionally long-lasting psychedelic and psychostimulating effects. Most users report the effects of this drug lasting at least 18 hours, with others suggesting effects finally pittering out as much as 36 hours later.
On top of its long duration of effects, DOB is also exceptionally strong, with the threshold dose listed by Shulgin at just 0.2 mg (200 micrograms) — which is about the same dose as LSD. The standard dose of DOB is around 0.75 mg (roughly equivalent to taking about 3 or 4 tabs of acid).
DOX compounds like DOB, along with lysergamides (such as LSD) and 25I-NBOMes, are the only psychedelic drugs active at doses small enough to fit on blotter paper.
DOB Specs:
| Chemical Name | 4-Bromo-2,5-dimethoxyamphetamine |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 8-24 hours |
| Estimated Threshold Dose: | 0.25 mg |
| Common Dose: | 0.5–1 mg |
| PubChem ID: | 62065 |
| CAS#: | 64638-07-9 |

IUPAC Name: 1-(4-bromo-2,5-dimethoxyphenyl)propan-2-amine
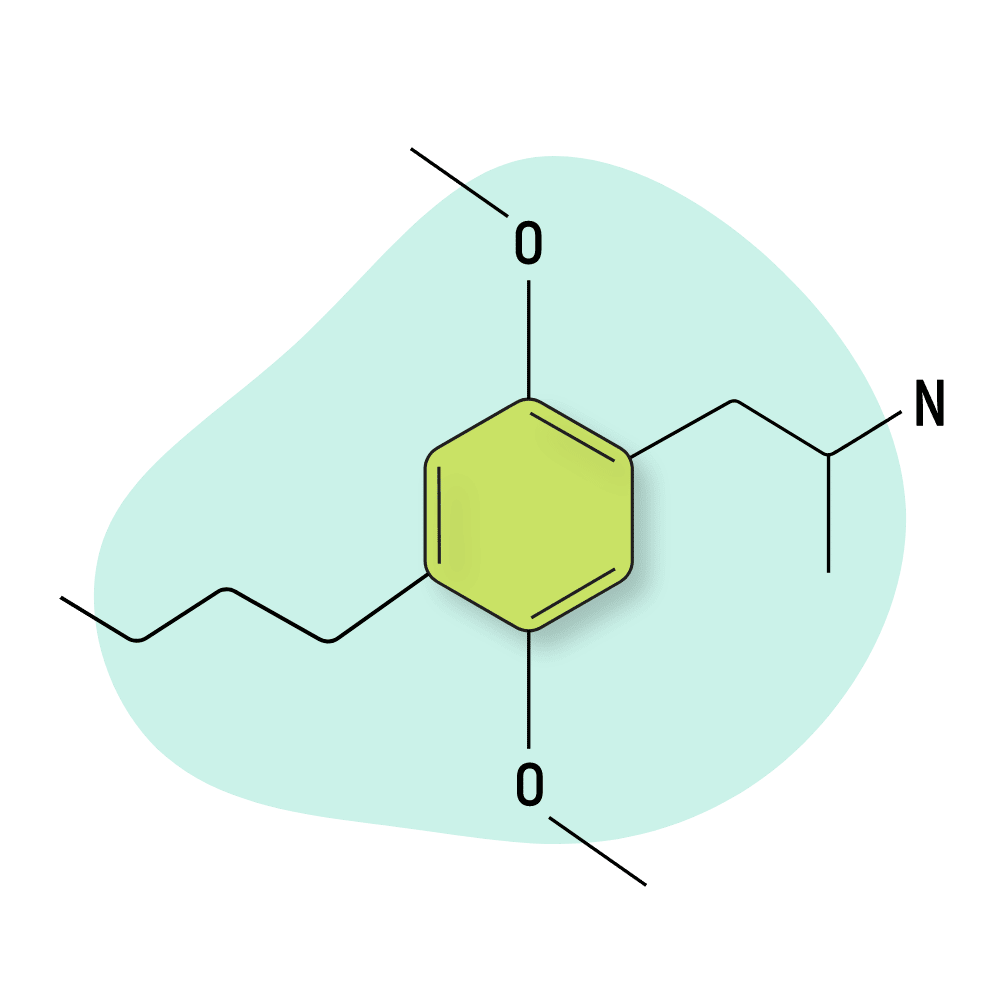
DOBU
DOBU (2,5-Dimethoxy-4-n-butylamphetamine) is the 4-carbon homolog of DOM, DOET, and DOPR. The effects of this research chemical are not well known, and this drug is not popular for recreational use.
Shulgin’s report on this compound suggests it has a very long come-up (over 4 hours before effects are felt) and a very long duration of action (over 18 hours). It produces a host of uncomfortable effects, such as manic intoxication, insomnia, and depressive hangover.
Sometimes this drug appears on designer drug marketplaces, but there isn’t much interest in this compound among recreational users.
DOBU Specs:
| Chemical Name | 2,5-Dimethoxy-4-n-butylamphetamine |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 18–36 hours |
| Estimated Threshold Dose: | <1 mg |
| Common Dose: | Unknown |
| PubChem ID: | 10060720 |
| CAS#: | 63779-89-5 |

IUPAC Name: 1-(4-butyl-2,5-dimethoxyphenyl)propan-2-amine
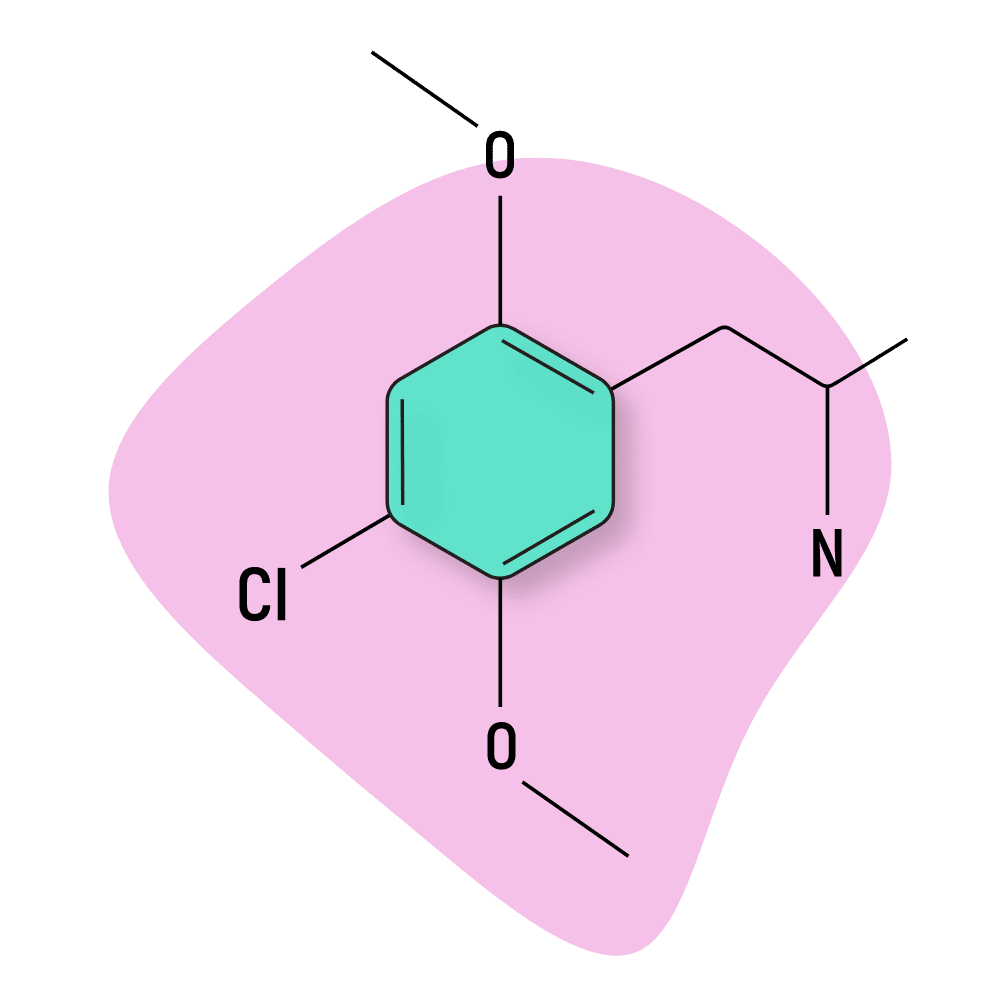
DOC
DOC (4-Chloro-2,5-dimethoxyamphetamine) is the amphetamine analog of the 2C psychedelic 2C-C.
DOC was first reported by researchers at the University of Alberta in 1973 [15] but wasn’t tested properly until much later by Alexander Shulgin. His reports in his book, PiHKAL, summed up the experience with “This is excellent stuff, but start early in the day.”
Like other DOX compounds, DOC is very potent (the standard dose is just 2–4 mg) and long-lasting (12–24 hours total). It can take several hours for this drug to even start kicking in. Do not double dose.
DOC Specs:
| Chemical Name | 4-Chloro-2,5-dimethoxyamphetamine |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 12–24 hours |
| Estimated Threshold Dose: | 0.5 mg |
| Common Dose: | 1.5–2 mg |
| PubChem ID: | 542036 |
| CAS#: | 123431-31-2 |

IUPAC Name: 1-(4-chloro-2,5-dimethoxyphenyl)propan-2-amine
DOEF
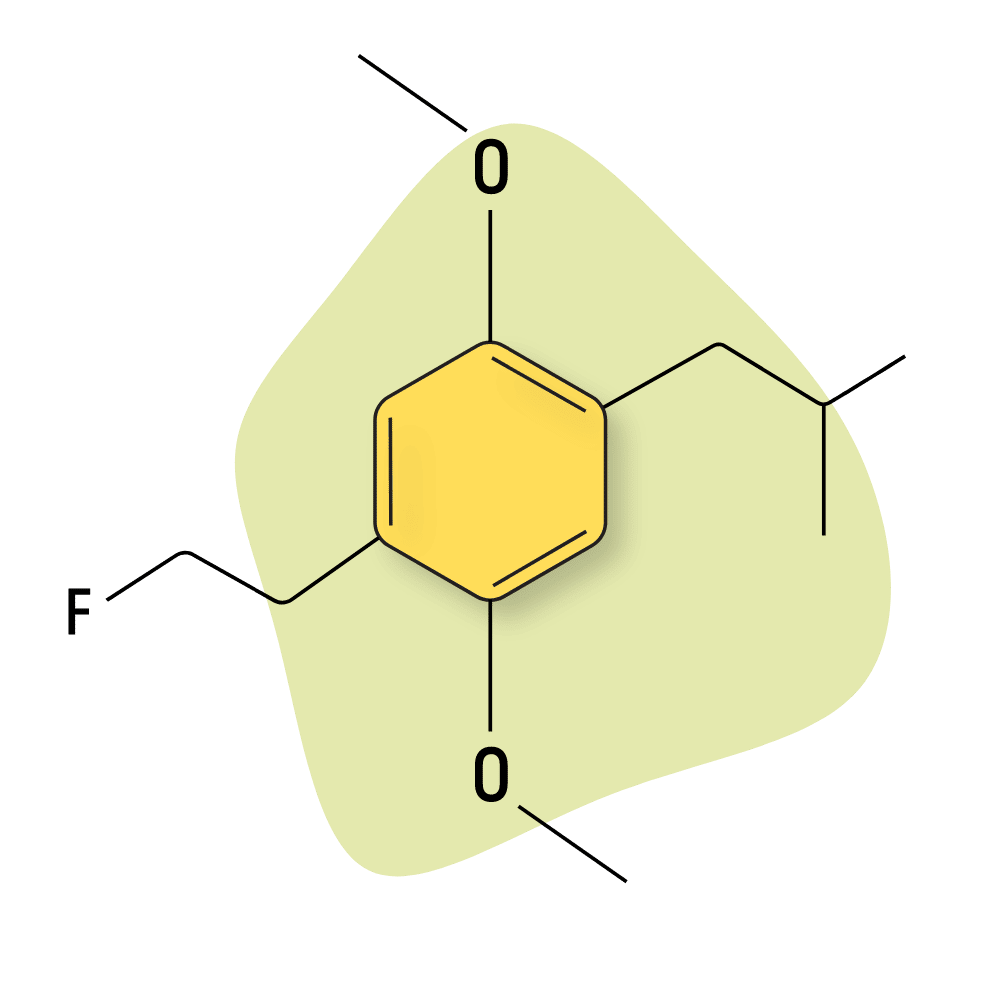
DOEF (2,5-Dimethoxy-4-(2-fluoroethyl)amphetamine) is not very popular — likely because of its lack of visual effects. Shulgin reports in PiHKAL that he created this drug by adding a fluorine atom in the hopes that he could one day use it to trace where this compound is depositing in the brain. To this day, studies of this nature have not been done, and DOEF remains an obscure and largely unused psychoactive compound.
Shulgin’s reports of DOEF suggested that it produced “dreams [that were] pointless.” He also notes a lack of both visual hallucinations and body load. Like most other DOX compounds, DOEF is very long-lasting, with Shulgin’s reports suggesting the trip lasts around 16 hours in total.
DOEF Specs:
| Chemical Name | 2,5-Dimethoxy-4-(2-fluoroethyl)amphetamine |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 12–16 hours |
| Estimated Threshold Dose: | 1 mg |
| Common Dose: | 2–3.5 mg |
| PubChem ID: | 14201982 |
| CAS#: | 121649-01-2 |

IUPAC Name: 1-[4-(2-fluoroethyl)-2,5-dimethoxyphenyl]propan-2-amine
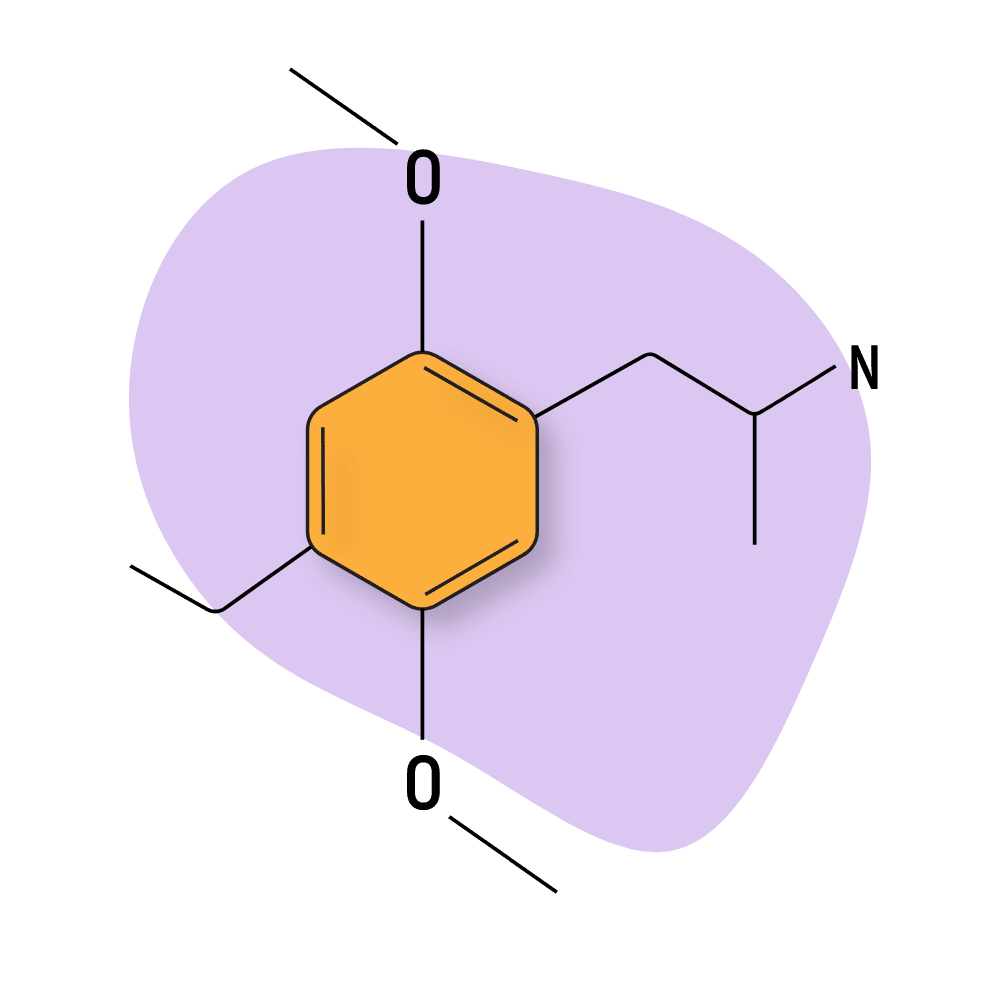
DOET
DOET (2,5-Dimethoxy-4-ethylamphetamine hydrochloride) is less potent than other DOX compounds, with the standard dose assumed to be around 6 or 7 milligrams, according to Shulgin. This compound is associated with powerful closed-eye visuals, mild open-eye visuals, and strong alterations in both smell and sound.
The mental state remains intact, and the body load tends to be the limiting factor for this psychedelic. Many people don’t like this compound because it can quickly lead to feelings of nausea and dizziness if too much is used.
DOET Specs:
| Chemical Name | 2,5-Dimethoxy-4-ethylamphetamine hydrochloride |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 14–20 hours |
| Estimated Threshold Dose: | 2 mg |
| Common Dose: | 4–6 mg |
| PubChem ID: | 62066 |
| CAS#: | 22139-65-7 |

IUPAC Name: 1-(4-ethyl-2,5-dimethoxyphenyl)propan-2-amine;hydrochloride
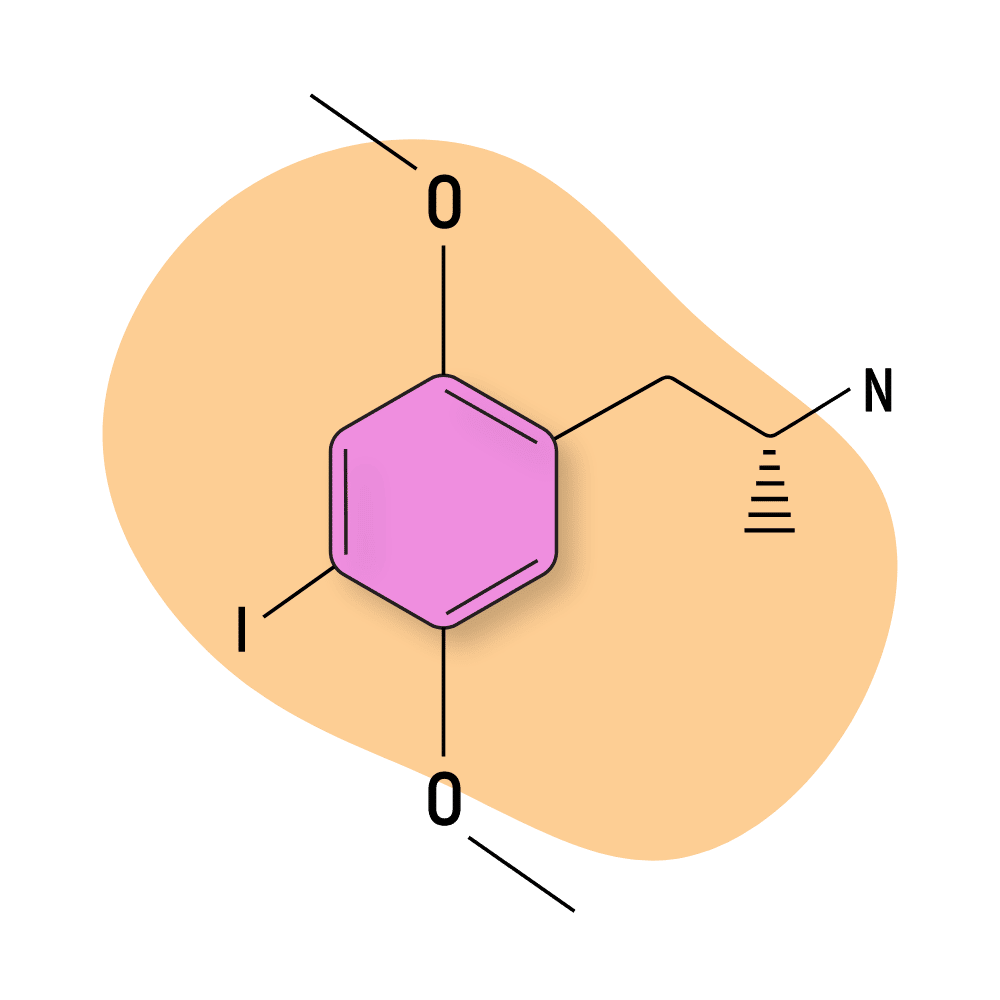
DOI
DOI (4-Iodo-2,5-dimethoxyphenylisopropylamine) is one of the strongest and most well-known members of the DOX family of compounds. This compound gets a lot of mixed reviews — some suggest the body load makes it unusable and it tends to produce feelings of depression and fear in a lot of users.
Others claim this compound to be one of the best amphetamine-based psychedelics around — producing feelings of bliss, strong, intriguing hallucinations, altered perception of time, and long-lasting effects. There have even been some studies on this compound that suggest promise as a tool in psychedelic-assisted psychotherapy for treating addiction [16].
DOI was officially added to the Controlled Substances List in the United States (along with DOC) in April 2022.
DOI Specs:
| Chemical Name | 4-Iodo-2,5-dimethoxyphenylisopropylamine |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 16–30 hours |
| Estimated Threshold Dose: | 0.5 mg |
| Common Dose: | 1–3 mg |
| PubChem ID: | 1229 |
| CAS#: | 64584-34-5 |

IUPAC Name: 1-(4-iodo-2,5-dimethoxyphenyl)propan-2-amine
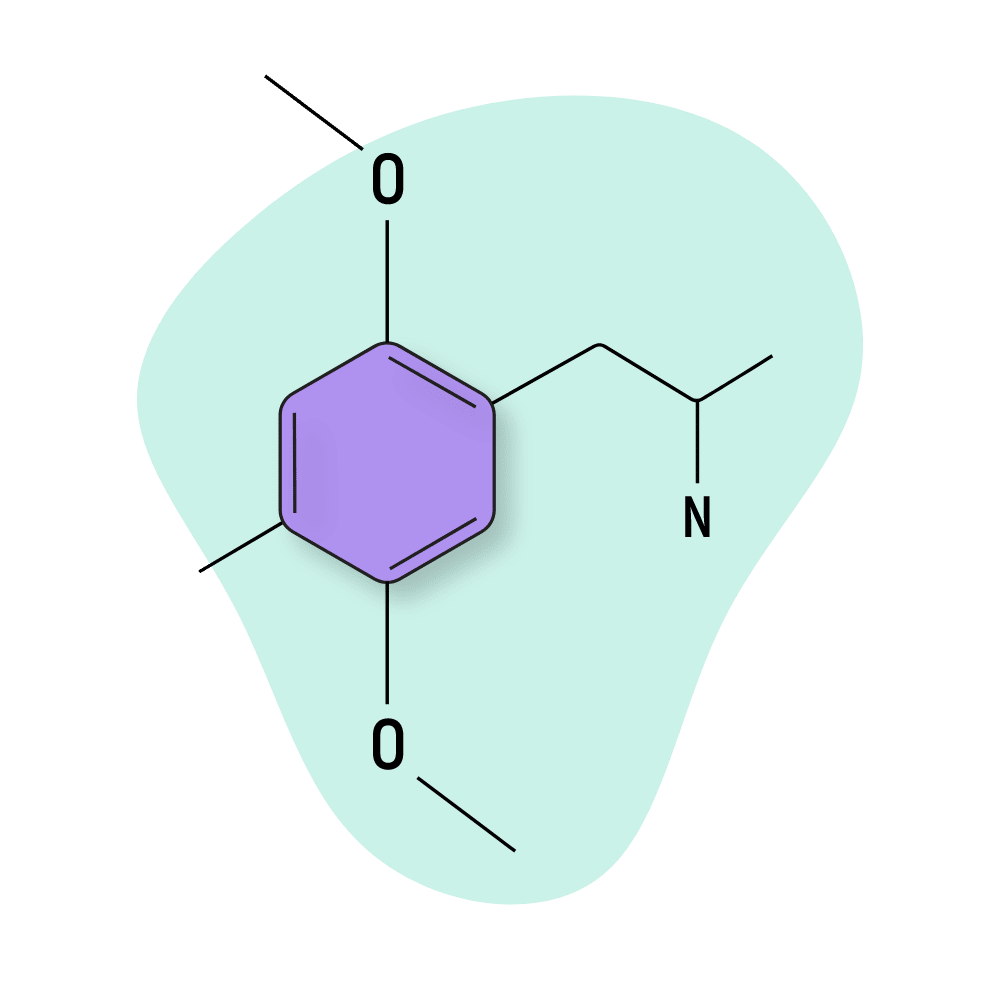
DOM
DOM (2,5-Dimethoxy-4-methylamphetamine) was listed as one of Shulgin’s so-called “magical half-dozen,” — which referred to the duo’s top six psychedelics ever created. DOM was the only DOX to make the list, along with mescaline and several members of the 2CX family.
This psychedelic can easily produce negative “eerie” feelings, as well as beautiful vibrant, and jubilant sensations depending on the surroundings — it’s extra important to be wary of set and setting, including who joins you on the journey. The effects of DOM remain active for nearly a full day, with some tapering-off effects felt throughout the following day as well.
DOM Specs:
| Chemical Name | 2,5-Dimethoxy-4-methylamphetamine |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 14–20 hours |
| Estimated Threshold Dose: | 1 mg |
| Common Dose: | 2.5–5 mg |
| PubChem ID: | 85875 |
| CAS#: | 15588-95-1 |

IUPAC Name: 1-(2,5-dimethoxy-4-methylphenyl)propan-2-amine
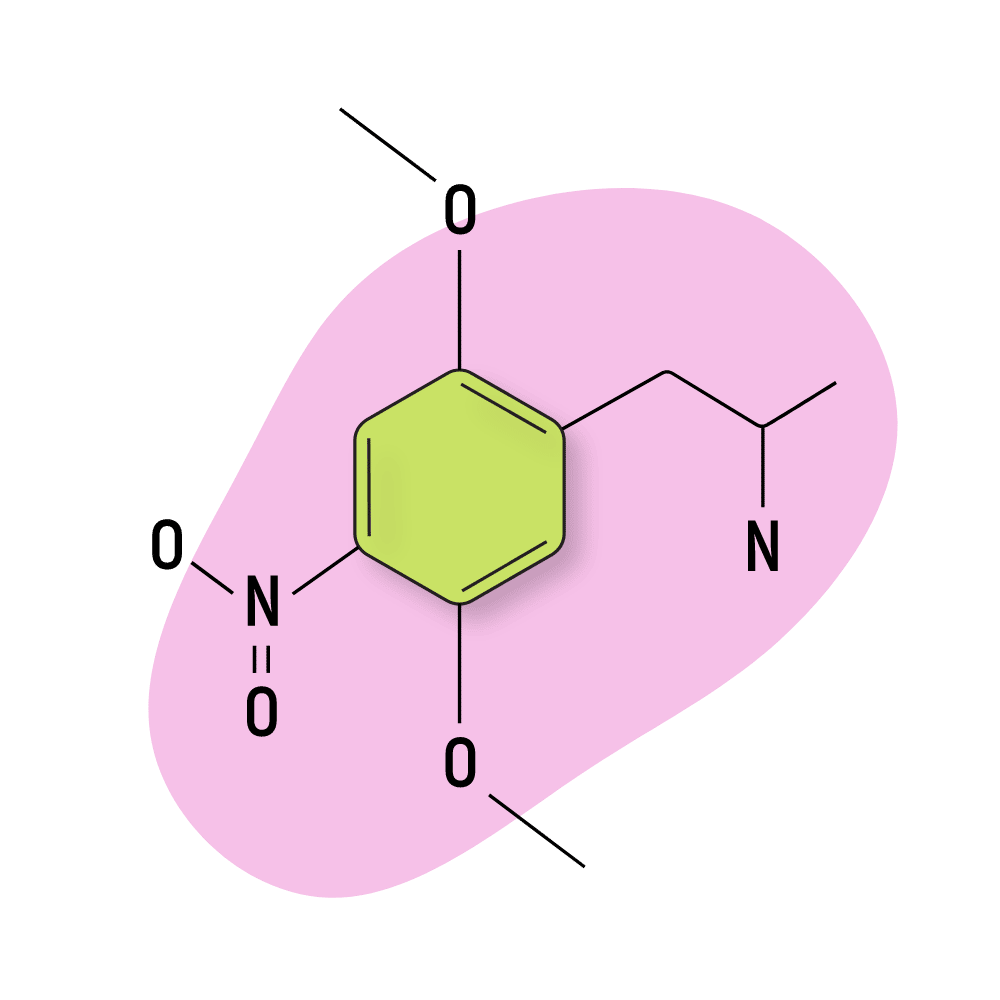
DON
DON (2,5-Dimethoxy-4-nitroamphetamine) is much less popular than DOI or DOM, but there are some people who report using this substance on Reddit and the Bluelight forums.
This is a good example of a psychedelic that’s only suitable for more experienced psychonauts.
While DON is strongly psychedelic, getting the dose right is notoriously difficult. There’s a balance between taking too little (which just feels stimulating and anxious) and too much (which has a tendency to produce hyperthermia and paranoia). Users who land the dose report feeling motivated and creative, with strong open and closed-eye visuals.
DON Specs:
| Chemical Name | 2,5-Dimethoxy-4-nitroamphetamine |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 8–15 hours |
| Estimated Threshold Dose: | 1 mg |
| Common Dose: | 3–5 mg |
| PubChem ID: | 105432 |
| CAS#: | 67460-68-8 |

IUPAC Name: 1-(2,5-dimethoxy-4-nitrophenyl)propan-2-amine
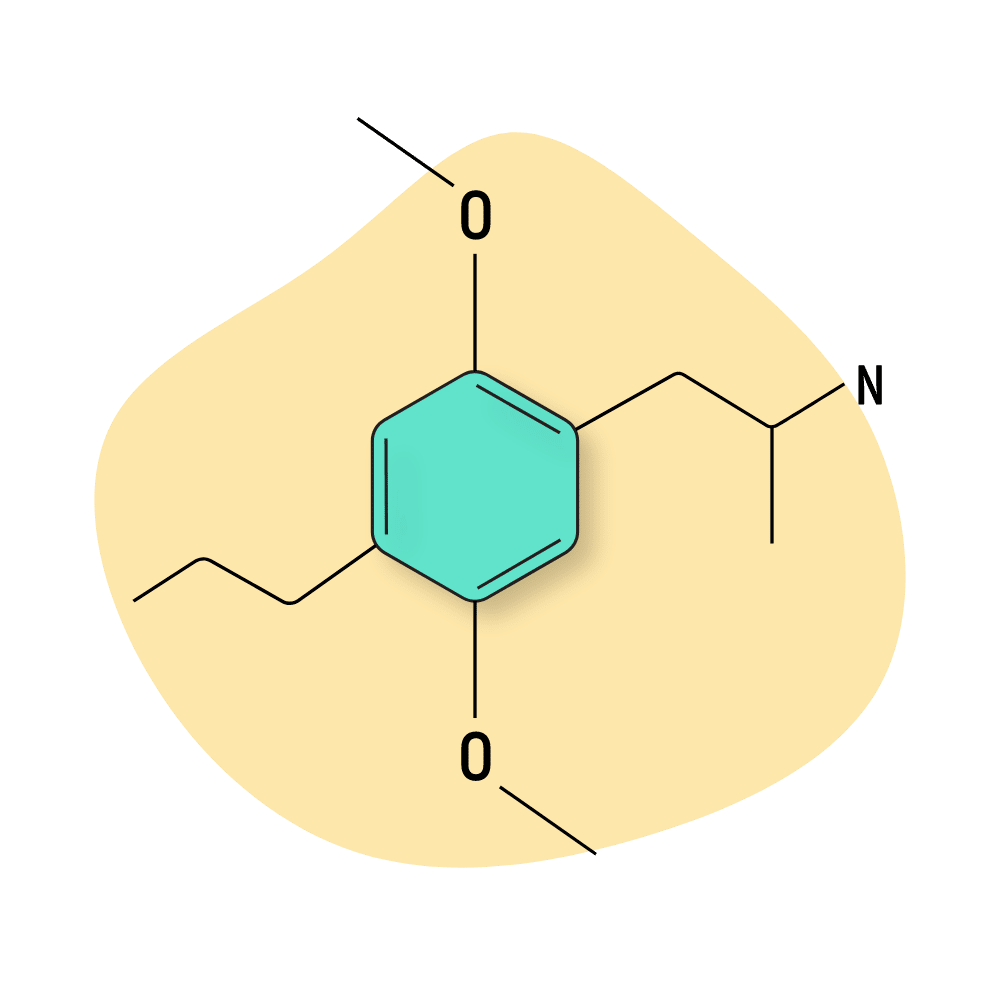
DOPR
DOPR (2,5-Dimethoxy-4-propylamphetamine) has a very slow onset of effects leading up to strong visual and stimulatory (amphetamine-like) effects. It has a high affinity for side effects like nausea, muscle tension, fear/worry, and mental scramble.
There’s not much information available about this substance, and it’s hard to find. This is not likely going to change anytime soon — many other DOX compounds produce reliably potent psychedelic and stimulating experiences that don’t risk a significant negative body load for nearly 30 hours straight.
DOPR Specs:
| Chemical Name | 2,5-Dimethoxy-4-propylamphetamine |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 20–30 hours |
| Estimated Threshold Dose: | 1.5 mg |
| Common Dose: | 2.5–3.5 mg |
| PubChem ID: | 542051 |
| CAS#: | 63779-88-4 |

IUPAC Name: 1-(2,5-dimethoxy-4-propylphenyl)propan-2-amine
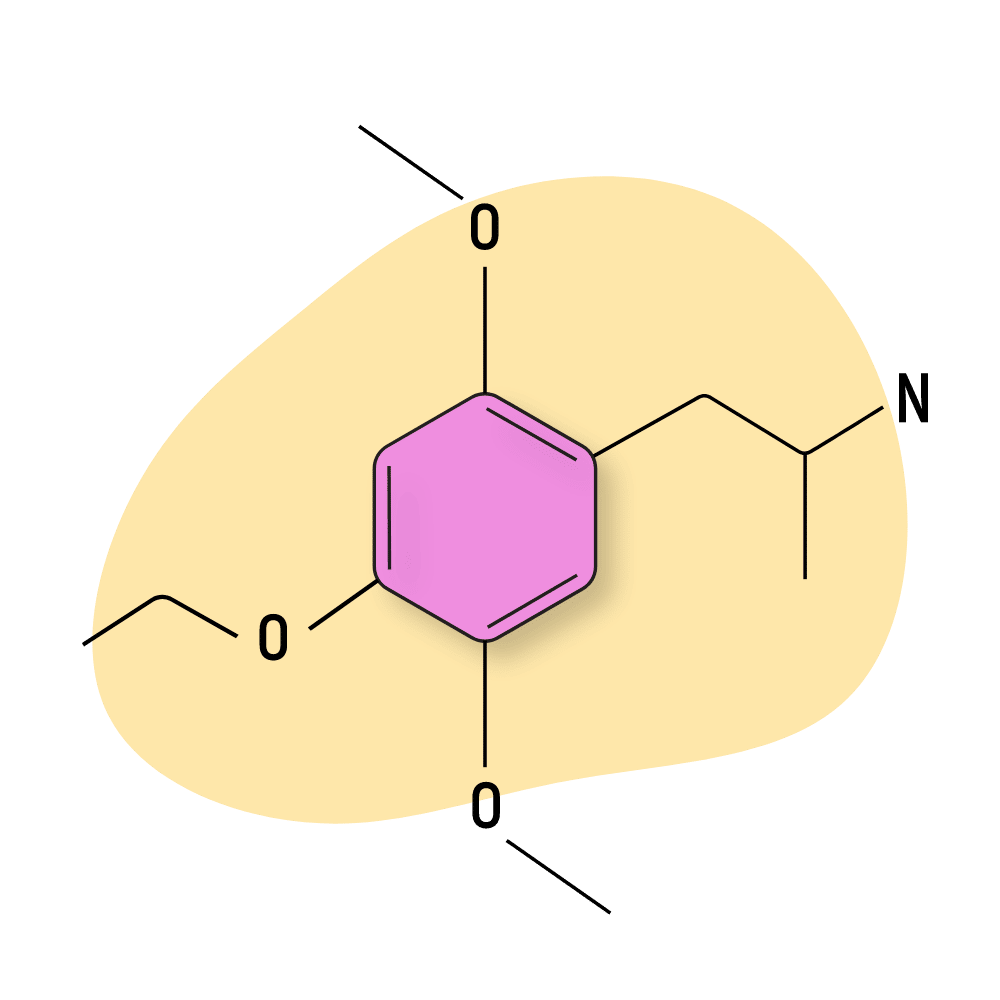
MEM
MEM (2,5-Dimethoxy-4-ethoxyamphetamine) isn’t a particularly strong compound. The effects are similar to amphetamines and produce feelings of euphoria and energy, along with some of the empathogenic qualities of MDMA (though to a much lesser extent).
Shulgin reports the primary value of this compound comes the following day in the form of enhanced inspiration and insight.
MEM Specs:
| Chemical Name | 2,5-Dimethoxy-4-ethoxyamphetamine |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 10–14 hours |
| Estimated Threshold Dose: | 10 mg |
| Common Dose: | 25–35 mg |
| PubChem ID: | 542053 |
| CAS#: | 16128-88-4 |

IUPAC Name: 1-(4-ethoxy-2,5-dimethoxyphenyl)propan-2-amine
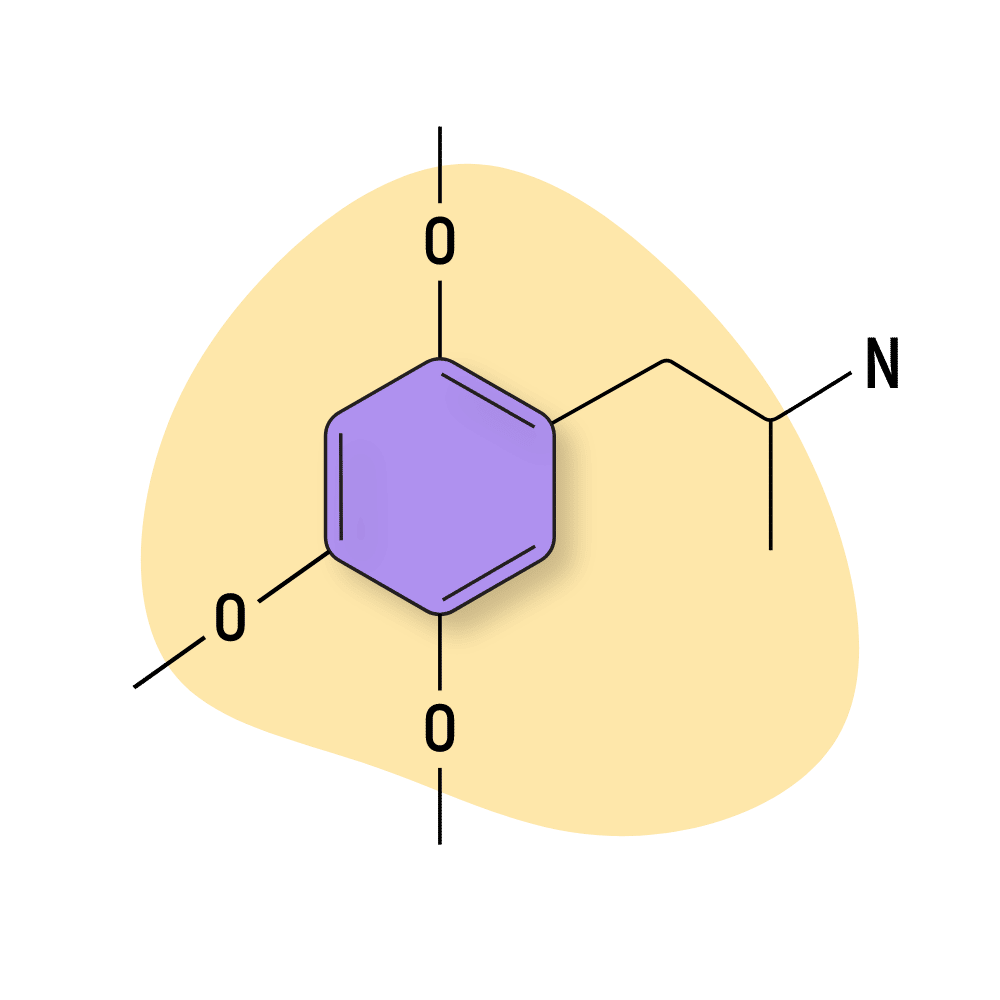
TMA-2
TMA-2 (2,4,5-Trimethoxyamphetamine) is just one of several compounds in the TMA group (TMA, TMA-2, TMA-3, TMA-4, TMA-5, and TMA-6).
TMA-2 is one of the rare amphetamine molecules associated with sedative effects rather than the classic stimulating action.
The psychedelic qualities of this compound become apparent at the 20 mg and above dose, producing potent alterations in the perception of time, closed and open-eye visuals, and kaleidoscopic imagery. Shulgin reports the effects to be “calmly cosmic and clear thinking.” It was one of the first psychedelics he ever created.
TMA-2 Specs:
| Chemical Name | 2,4,5-Trimethoxyamphetamine |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 8–12 hours |
| Estimated Threshold Dose: | 10 mg |
| Common Dose: | 20–40 mg |
| PubChem ID: | 31014 |
| CAS#: | 1083-09-6 |

IUPAC Name: 1-(2,4,5-trimethoxyphenyl)propan-2-amine
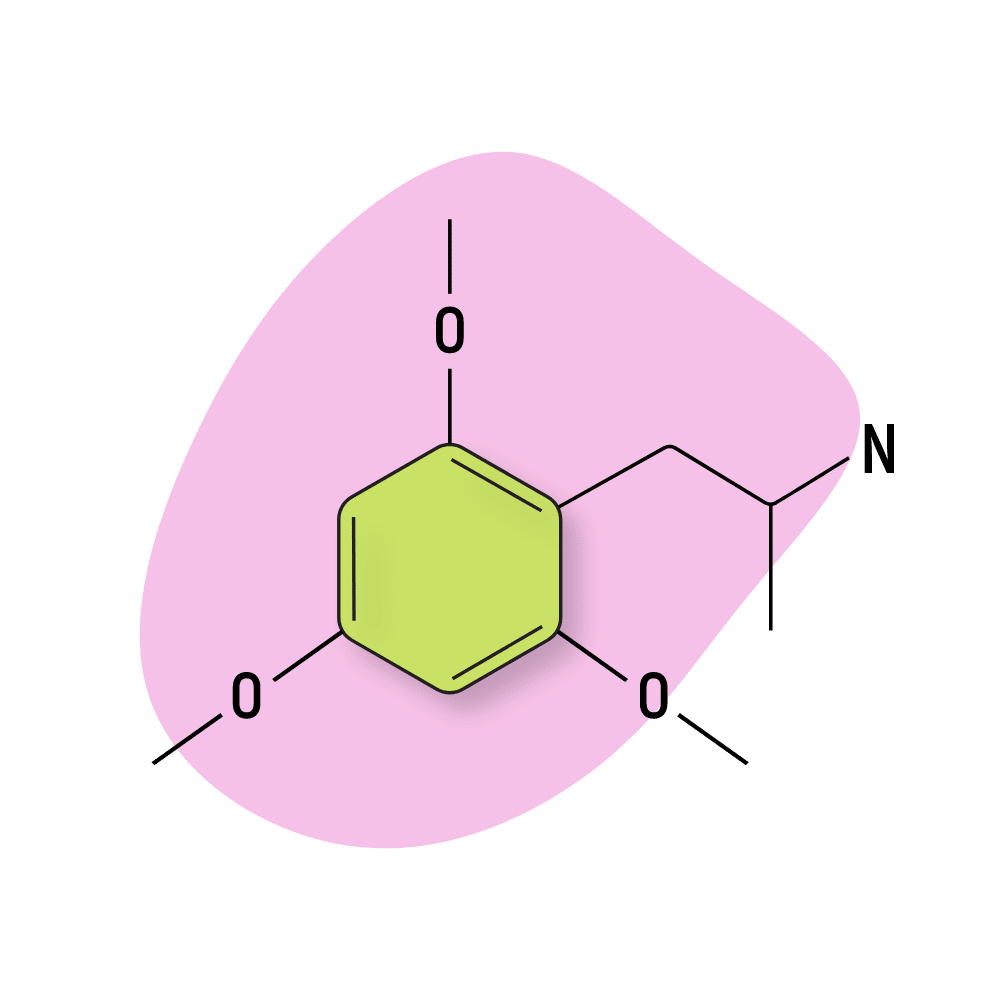
TMA-6
TMA-6 (2,4,6-Trimethoxyamphetamine) is the other psychoactive TMA compound (along with TMA-2) and is exceptionally potent, just like its counterpart. TMA-6 is very intoxicating — making it hard to think straight or accomplish even simple tasks without outside help. Shulgin reported this compound as being “extremely funny” and found it hard not to laugh pretty much nonstop throughout the experience.
This compound is cheap to produce, and the potent, long-lasting psychoactive effects allow it to compete with compounds like DOB and DOI.
TMA-6 Specs:
| Chemical Name | 2,4,6-Trimethoxyamphetamine |
| Status: | Research Chemical 🧪 |
| Duration of Effects: | 12–16 hours |
| Estimated Threshold Dose: | 15 mg |
| Common Dose: | 30–40 mg |
| PubChem ID: | 31015 |
| CAS#: | 15402-79-6 |

IUPAC Name: 1-(2,4,6-trimethoxyphenyl)propan-2-amine
Natural Amphetamines
The vast majority of substituted amphetamines are synthetic and do not occur in nature — but there are a few exceptions.
Two species of plants are a particularly abundant source of amphetamines — khat (Catha edulis) and ephedra (Ephedra sinica). Most of the designer drugs in circulation today are based on the compounds produced by these plants directly or are derivatives of derivatives.
1. Ephedra sinica (Ma Huang)
Ephedra (AKA Ma Huang) can be found growing on every continent, with the exception of Antarctica. It’s a very old species of plant — one of just three living members of the Gnetophyta family of plants that dates back to the cretaceous period (65–144 million years ago).
Ephedra is a natural source of several amphetamines, including ephedrine, pseudoephedrine (cathine), and norephedrine (phenylpropanolamine).
In Traditional Chinese Medicine, Ma Huang is used as a treatment for asthma, nasal congestion, and bronchitis — which owes itself to the adrenergic stimulating qualities of these amphetamines.
More modern uses of the plant include weight loss and pre-workout.
The phyto-amphetamines in ephedra have also been used to manufacture methamphetamines by clandestine labs, leading to the banning of ephedra in the US in 2004.
2. Catha edulis (Khat)
The Khat plant is a small flowering plant from the Middle East and Northern Africa that has been used as a traditional medicine and recreational stimulant for thousands of years.
The active ingredients in khat are cathine and cathinone — two potent amphetamines. These compounds are the starting point for the modern era of synthetic cathinone stimulants such as alpha-PVP, MDPV, and mephedrone.
Unlike ephedra, khat doesn’t have a lot of therapeutic uses, even within the traditional cultures that use the herb. This plant is almost exclusively recreational. Users chew the fresh leaves or prepare a strong tea from the dried leaves. Khat chewing/drinking is a common social practice among many cultures living in the Horn of Africa, where the khat plant thrives.
Today, khat chewing has spread around the world. The cost of khat has increased substantially, so many farmers living in Yemen next to the Red Sea have stopped growing fruits and vegetables to focus on khat instead — a crop that produces an average of 3X the value of other commercial crops. Khat production accounts for a whopping 30% of Yemen’s economy and 40% of the country’s water consumption.
How Do Amphetamines Work?
Each of our neurons has a collection of receptors located within the synapses. A synapse is the point of contact between neurons where information is passed. Neurons pass this information in the form of chemical messengers called neurotransmitters such as dopamine, norepinephrine, or serotonin.
Once a neurotransmitter is passed from one neuron to the next, it binds to a receptor and causes an effect. Once finished, it’s reabsorbed into the neuron, where it’s broken down and recycled.
Amphetamines work by altering one or more of these processes — leading to an accumulation of neurotransmitters and an increased excitatory (stimulated) state.
There are three main mechanisms by which amphetamines cause their stimulating effects:
- Some block the reuptake of neurotransmitters
- Some block the clearance of neurotransmitters
- Some reverse the polarity of neurotransmitter transporters
All three mechanisms accomplish more or less the same thing — an overall increase in neurotransmitter levels within the brain.
Each drug is different and has a different receptor affinity — some compounds focus more on serotonin (such as MDMA and MDA), some more on dopamine (most of the cathinones), and others on norepinephrine (Bupropion). No drug is specific to one type of neurotransmitter — each one targets a slurry of receptors in different ways. This is what gives each amphetamine its distinct set of effects.
The Dangers of Amphetamines
Like all drugs, no amphetamine is inherently good or bad. Many have clear medicinal uses, and many others (often the same ones) can also be deadly. The only difference between these two applications is the dosage.
Most of the deaths or significant health issues resulting from amphetamines can be traced back to problems associated with prohibition. This includes undefined potency, adulteration, and lack of understanding of how to use the drug safely — leading to problems with mixing drug combinations and interactions or taking them while in an unsafe environment.
When used at the right dose, in a safe setting, and with people you trust, amphetamines can be incredibly useful tools for self-exploration and development. The key here is to know what you’re using, understand the dose, take measures to test the substance you’re using, and don’t overuse them.
Psychedelic amphetamines are making a comeback into mainstream healthcare recently as well as more clinical studies are published each year exposing the powerful health benefits of substances like MDMA and related amphetamines.
1. Amphetamine Toxicity
Any drug that alters brain chemistry carries risks. Amphetamines are no exception, but they aren’t as dangerous as the media has made them out to be either.
Let’s break down the differences between the short-term and long-term dangers of amphetamines.
Acute Toxicity (Overdose)
Very high doses of amphetamines are toxic — regardless of the substance. However, some compounds are much more dangerous than others. Drugs with a low threshold dose (such as many synthetic cathinones or PMA/PMMA) or drugs that can be smoked or injected (such as methamphetamine) pose the most substantial risk for overdose.
Overdosing on amphetamines is serious business. According to the CDC, nearly a quarter of all overdose-related deaths in 2019 were the result of psychostimulants such as amphetamines. This accounted for a 28% increase compared to 2018. One of the reasons for this is the growth of very high-potency psychostimulants like synthetic cathinones, which only entered the market in the mid-2000s. Just one puff too many of something like alpha-PVP can result in a deadly overdose.
Toxic levels of amphetamines lead to widespread damage to the cardiovascular and neurological systems. High blood pressure, mixed with rapid and irregular heart rate and dehydration, can cause a heart attack or stroke; excessive serotonin or dopamine in the brain can cause seizures, neuronal death, and coma.
The most common signs of amphetamine overdose include:
- Chills & shaking
- Hyperthermia (high fever)
- Dehydration
- Burning urination
- High blood pressure
- Arrhythmia & tachycardia (irregular & rapid heart rate)
- Psychosis & delusions
- Restlessness or erratic behavior
- Nausea & vomiting
- Loss of consciousness
Chronic (Long-Term) Toxicity & Abuse
Amphetamine abuse is associated with problems with addiction, neurotoxicity, hepatotoxicity (liver damage), and psychosis. In a similar capacity to other classes of drugs, such as the arylcyclohexylamines (like PCP or ketamine), long-term amphetamine abuse often leads users to develop delusions that don’t make sense to anybody but themselves.
According to the National Survey on Drug Use and Health (NSDUH), nearly 6 million people abused amphetamines (both legal and illegal) in 2015. The most common amphetamine of abuse is methamphetamine, followed by synthetic cathinones (Bath Salts) such as mephedrone, alpha-PVP, and MDPV.
2. Amphetamine Addiction
Amphetamines are addictive because they operate on two specific mechanisms of addiction:
A) Behavioral Addiction (Using Drugs To Mask or Solve Other Problems)
Amphetamines offer three main effects — increased mental and physical energy, improved mood and motivation, and they help users focus and pay attention.
A drug’s ability to control specific elements of cognition can become addictive to people who identify with problems related to those areas of cognition (such as attention, libido, energy levels, mood, etc.). Most addiction experts consider the problem of addiction to be more related to what the drug “does” for someone rather than focusing on what kind of brain abnormalities may be present.
For example, someone who has a hard time focusing at work or university may seek a drug like Adderall because it can solve this problem for them. Others may use something like MDMA because it helps them temporarily escape feelings of unhappiness or depression.
B) Physical Addiction (Tolerance & Altered Neurochemical Balance)
Physical addiction takes longer to develop but is much harder to fix. When someone repeatedly takes a drug that alters neurotransmitter levels in any way, the body starts to compensate for the drug. There’s a reason our dopamine levels aren’t constantly at 200%, so the body starts to hide or alter dopamine receptors or simply produces less dopamine to maintain as close to baseline as possible despite the presence of the drug.
When someone takes amphetamines often, they’ll need to keep increasing the dose in order to feel the same intensity of effects. This is tolerance.
Once they stop taking the drug, they begin to feel sick. The very problems the drug offers to fix come back with a vengeance. People feel lethargic, depressed, and unable to focus. They may experience physical side effects like increased pain sensitivity or nausea. The only thing that makes these symptoms go away is more of the drug.
Over time, these withdrawal symptoms disappear, but the process is very uncomfortable. People who are severely addicted to amphetamines may need to spend time in a detox center. While there, doctors can administer gradually decreasing doses of amphetamines until the body is able to achieve balance.
3. Amphetamine Adulteration
One of the byproducts of prohibition is a tainted drug supply. The clandestine labs manufacturing these compounds often mix cheaper, more dangerous, or addictive compounds into their products to reduce the bottom line and drive more profit.
Some adulterants are highly toxic — such as PMA, PMMA, NBOMes, or opiates like fentanyl and carfentanyl. Some adulterants aren’t necessarily toxic if used properly but can be deadly if mistaken for something else that normally requires a higher dose — MDMA adulterated with synthetic cathinones is a good example of this.
When you buy an illegal drug of any kind, assume it’s adulterated until proven safe. Nothing short of lab testing is going to confirm the purity of your drugs, but you can run simple and cost-effective tests to rule out the most problematic substances.
It’s wise to keep some drug reagent test kits around if you plan on taking amphetamines like MDMA or black market Adderall. These kits cost less than $40 and provide enough for hundreds of tests (more on DIY testing below).
Harm Reduction: How to Test Your Amphetamines
Testing a small sample of your substance using a chemical reagent test liquid (or the newer solid reagent test chemicals) is considered a key element of using these substances responsibly.
There’s no good reason not to — you only need a sample the size of a grain of sand to run the test, and test kits are very cheap (much, much cheaper than the drugs they’re being used to test).
Regardless of your source, it’s wise to assume all amphetamines and amphetamine-derivatives are adulterated until proven otherwise. If people like Mac Miller — who we can assume was not limited by the higher cost of pure drugs — can accidentally die from adulterated drugs, anybody can.
When it comes to amphetamine testing, the main targets to look for are opiates (there shouldn’t be any in there whatsoever, so the type or amount doesn’t matter much), PMA/PMMA, and NBOMEs. You’re also looking to confirm that the type of amphetamine you think is in there is actually in there (because the dose can vary a lot).
When testing amphetamines, you should use the following reagents:
- Marquis
- Mecke
- Robadope
- Simon’s Reagent
Step 1: Break Off or Separate A Small Sample of the Substance
If you have a tablet, scrape a small amount (grain of sand worth) using a sharp knife. Place the sample on a light-colored, inert surface. A white ceramic plate or the bottom of a mug works great for this.
You will need a separate sample for each test. Make sure to separate each test from the others so the reagents don’t mix together.
Step 2: Place One Drop of the Test Substance On To the Sample
For the case of Simon’s, you’ll need one drop each of reagent part one and reagent part 2.
Step 3: Give the Sample Time to React & Compare the Results To the Chart Included With Your Kit
Reagents work by changing color in the presence of certain types of compounds. This means they can detect the presence of a chemical, but they won’t tell you the exact amount. Using multiple tests like this allows you to rule out or in certain chemicals.
For example, if you have pure MDMA, your results should go something like this:
- Marquis → Dark purple
- Mecke → Black
- Rhobadope → No color change
- Simon’s → Dark-Blue
Each test kit should come with its own color change guide, so use the one that comes with your kit. If your kit didn’t come with one, you should be able to find one on the website or follow the guide below.
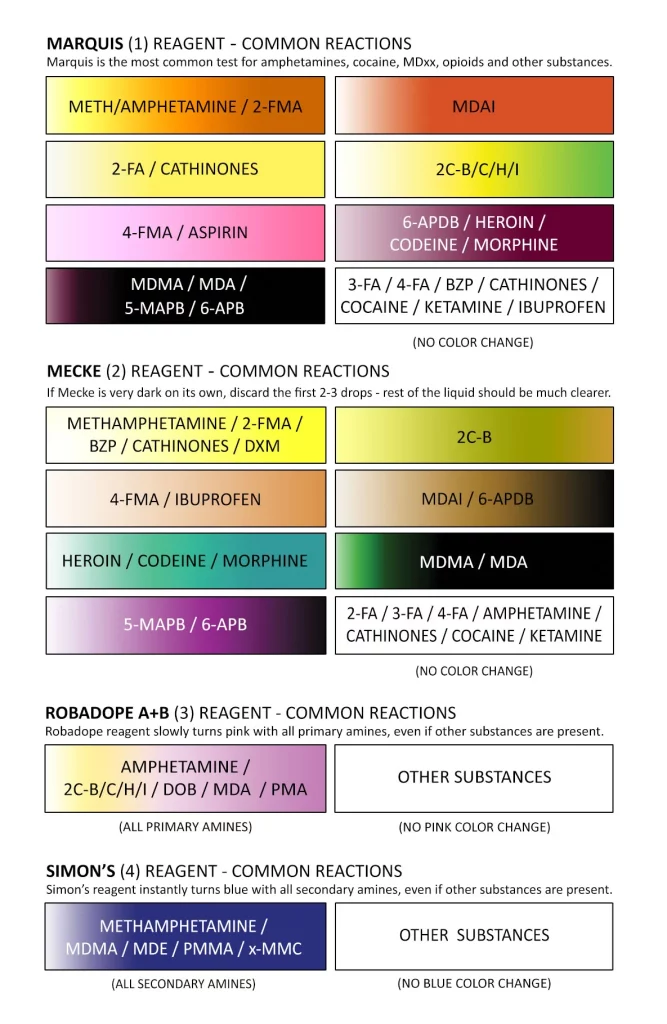
(Source: ProTest Kit)
Amphetamines Legal Status
Most amphetamines are either Schedule I or Schedule II substances (or their equivalents) in the US, Canada, Australia, and the European Union.
The psychedelic amphetamines, including MDMA, MDA, synthetic cathinones, and most DOX compounds, are directly banned, earning prohibition status as recently as April 2022 (DOM and DOB).
The US, Germany, France, and various members of the EU ban all other DOX compounds through the existence of analog acts. Analog acts provide a blanket ban on any new psychoactive compound that shares a similar structure to a known restricted substance.
Canada is one exception where these laws don’t exist. Many DOX amphetamines remain legal here as a result.
Some amphetamines, such as amphetamine, dextroamphetamine, and lisdexamfetamine, are available as prescription-only medications. These drugs treat diagnosed medical conditions such as ADHD, Parkinson’s Disease, and narcolepsy.
A Brief History of Amphetamines
Plants containing amphetamines have been used for thousands of years as medicine. Ephedra is used in Chinese medicine for treating asthma and breathing difficulties, and khat is used in social gatherings or as a stimulant to help laborers work longer hours before tiring.
Amphetamine was first synthesized in 1887 by Lazăr Edeleanu — a Romanian chemist. However, it wasn’t tested or used in medical practice until the early 1930s. The first medical applications involved the treatment of asthma, bronchitis, and cold and flu symptoms (as a decongestant). It was later adopted as a treatment for migraines, narcolepsy, epilepsy, Parkinson’s disease, and fevers.
Most famously, amphetamine was used in World War II by the Germans as a way to keep their soldiers awake and alert during the war.
The rise of substituted amphetamines didn’t begin until the 1960s, during the counterculture movement. In 1976, infamous psychedelic chemist, Alexander Shulgin, discovered a new way to synthesize MDMA that got around patents for the compound owned by Merck Pharmaceuticals. Shulgin and his followers popularized MDMA for both medical and recreational use, eventually leading to the banning of MDMA and related amphetamines in 1985.
Starting around the year 2000, the number of substituted amphetamines grew substantially as drug makers raced to create new compounds to sell as “legal highs” before regulators stepped in to ban them.
There are now thousands of designer drugs grouped within the substituted amphetamine class, including various sub-families of psychedelic amphetamines such as DOX compounds, synthetic cathinones, MDXX, and more.
Amphetamines FAQs
1. What is the Strongest Amphetamine?
The DOX compounds are among the strongest amphetamines ever discovered. They’re the only group of substituted amphetamines that are active at the sub-milligram dose. Because of how potent these compounds are, they’re usually sold in blotter paper form rather than in capsules or powder. Compounds such as DOB, DOI, DOC, DOPR, DOEF, and DON are all psychoactive in doses around 1 mg or less.
Synthetic cathinones are another powerful group of amphetamines. Compounds such as MDPV, α-PPP, and α-PVP are all active around the 3–5 mg mark.
Basic amphetamines like Selegiline, Xylopropamine, and methamphetamine are all active in doses starting around 10 mg.
Fluorinated amphetamines like 2-FA, 3-FA, and 3-FMA are active in the 15 mg range.
The MDXX group, which is home to MDMA and MDA, is the least potent overall — most of the compounds in this group aren’t active until roughly the 50 mg mark.
2. How Long Are Amphetamines Detectable In Urine?
Each amphetamine has a different half-life, which describes the amount of time it takes for half the substance to be removed from the body. Most amphetamines are eliminated through urine, so traces of the compound may remain detectable for as long as the drug remains in the system.
Most amphetamines are undetectable in the urine within 1 week. Some, such as Adderall (amphetamine), have a very short half-life and may be eliminated as quickly as 24 hours.
3. What Can I Do If I’m Addicted to Amphetamines?
Addiction to stimulant drugs is common — you are not alone. The first step to getting help is to reach out to either a doctor, a family or friend or one of the addiction hotlines listed below.
Depending on where you live, it may take some time to get treatment. It’s wise to start the process now, even if you don’t think you’re completely ready to quit yet.
Kicking amphetamine addiction isn’t easy, but there are services available to help reduce the discomfort of withdrawals and get you back on your feet — you just have to ask for it.
Addiction hotlines:
- USA — 1 800 662 4357
- Canada — 1 866 585 0445
- United Kingdom — 0300 999 1212
If you are in an emergency situation, call 911 or go to your local emergency department immediately.
References
- Baumann, M. H., Walters, H. M., Niello, M., & Sitte, H. H. (2018). Neuropharmacology of synthetic cathinones. In New Psychoactive Substances (pp. 113-142). Springer, Cham.
- Baumann, M. H., Partilla, J. S., Lehner, K. R., Thorndike, E. B., Hoffman, A. F., Holy, M., … & Schindler, C. W. (2013). Powerful cocaine-like actions of 3, 4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology, 38(4), 552-562.
- Baumann, M. H. (2014). Awash in a sea of ‘bath salts’: implications for biomedical research and public health. Addiction (Abingdon, England), 109(10), 1577.
- Ray, A., Chitre, N. M., Daphney, C. M., Blough, B. E., Canal, C. E., & Murnane, K. S. (2019). Effects of the second-generation” bath salt” cathinone alpha-pyrrolidinopropiophenone (α-PPP) on behavior and monoamine neurochemistry in male mice. Psychopharmacology, 236(3), 1107-1117.
- Maier, J., Rauter, L., Rudin, D., Niello, M., Holy, M., Schmid, D., … & Sitte, H. H. (2021). α-PPP and its derivatives are selective partial releasers at the human norepinephrine transporter: a pharmacological characterization of interactions between pyrrolidinopropiophenones and high and low affinity monoamine transporters. Neuropharmacology, 190, 108570.
- Hyde, J. F., Browning, E., & Adams, R. (1928). Synthetic homologs of d, l-ephedrine. Journal of the American Chemical Society, 50(8), 2287-2292.
- Oh, J. H., Hwang, J. Y., Hong, S. I., Ma, S. X., Seo, J. Y., Lee, S. Y., … & Jang, C. G. (2018). The new designer drug buphedrone produces rewarding properties via dopamine D1 receptor activation. Addiction Biology, 23(1), 69-79.
- Costa, R., Oliveira, N. G., & Dinis-Oliveira, R. J. (2019). Pharmacokinetic and pharmacodynamic of bupropion: integrative overview of relevant clinical and forensic aspects. Drug metabolism reviews, 51(3), 293-313.
- Kalix, P. (1992). Cathinone, a natural amphetamine. Pharmacology & Toxicology, 70(2), 77-86.
- Oeri, H. E. (2021). Beyond ecstasy: Alternative entactogens to 3, 4-methylenedioxymethamphetamine with potential applications in psychotherapy. Journal of Psychopharmacology, 35(5), 512-536.
- McKenna, D. J., Guan, X. M., & Shulgin, A. T. (1991). 3, 4-Methylenedioxyamphetamine (MDA) analogues exhibit differential effects on synaptosomal release of 3H-dopamine and 3H-5-hydroxytryptamine. Pharmacology Biochemistry and Behavior, 38(3), 505-512.
- Zhang, Z., An, L., Hu, W., & Xiang, Y. (2007). 3D-QSAR study of hallucinogenic phenylalkylamines by using CoMFA approach. Journal of Computer-Aided Molecular Design, 21(4), 145-153.
- Crean, R. D., Davis, S. A., Von Huben, S. N., Lay, C. C., Katner, S. N., & Taffe, M. A. (2006). Effects of (±) 3, 4-methylenedioxymethamphetamine,(±) 3, 4-methylenedioxyamphetamine and methamphetamine on temperature and activity in rhesus macaques. Neuroscience, 142(2), 515-525.
- Cozzi, N. V., Sievert, M. K., Shulgin, A. T., Jacob III, P., & Ruoho, A. E. (1999). Inhibition of plasma membrane monoamine transporters by β-ketoamphetamines. European journal of pharmacology, 381(1), 63-69.
- Coutts, R. T., & Malicky, J. L. (1973). The synthesis of some analogs of the hallucinogen 1-(2, 5-dimethoxy-4-methylphenyl)-2-aminopropane (DOM). Canadian Journal of Chemistry, 51(9), 1402-1409.
- Oppong-Damoah, A., Curry, K. E., Blough, B. E., Rice, K. C., & Murnane, K. S. (2019). Effects of the synthetic psychedelic 2, 5-dimethoxy-4-iodoamphetamine (DOI) on ethanol consumption and place conditioning in male mice. Psychopharmacology, 236(12), 3567-3578.
- Luethi, D., Kolaczynska, K. E., Docci, L., Krähenbühl, S., Hoener, M. C., & Liechti, M. E. (2018). Pharmacological profile of mephedrone analogs and related new psychoactive substances. Neuropharmacology, 134, 4-12.
- Inoue, T., & Suzuki, S. (1987). The metabolism of dimethylamphetamine in rat and man. Xenobiotica, 17(8), 965-971.
- Harris, S. C., & Worley, R. C. (1957). Analgesic properties of xylopropamine. Proceedings of the Society for Experimental Biology and Medicine, 95(2), 212-215.
