Could Psychedelics Treat Alzheimer’s Disease? Research Suggests It’s Possible
We aren’t implying psychedelics are the answer to everything, but they sure seem to hold a lot of potential — including possibly treating Alzheimer’s 💪
As I write this, my grandmother (in-law, but 100% still mine) is sitting near me, watching a game show — Make a Deal, if you’re curious, since we couldn’t find Wheel of Fortune.
She — and the rest of us — are mourning the recent death of her husband of 65 years, who died from Alzheimer’s.
A once strong man who worked hard for his family. Someone who loved his wife, kids, grandkids, and great-grandkids fiercely. Someone I would make snarky remarks to, and he would make them back, and laugh, saying he loved how we could joke around like that.
At least, that’s how it was until Alzheimer’s slowly started to take over.
As the years went by, he became shockingly thin, lost the ability to care for himself, and could barely move out of his chair. This from a man who almost never missed a day of work and took great pride in everything he did.
In some ways, we’re luckier than many. He never fully forgot his family. He always asked where his wife was when he couldn’t see her. He knew who his kids and grandkids were, mostly.
There were times he mistook his daughter for his sister-in-law or his grandson for the store employee, but if any of us asked if he knew who we were, he answered correctly and in a way that made us feel almost ludicrous for suggesting otherwise.
So, this topic is extremely personal to me, and likely is for many of you.
Just like with depression and PTSD, chances are most of us know someone struggling and would love to see something — anything — that can help bring these horrible diseases under control, offering hope to those suffering and a future to those not yet affected.
Could Psychedelics Prevent or Cure Alzheimer’s Disease?
Most research regarding psychedelics looks at how these substances can help reduce the neuropsychiatric symptoms (NPS) of Alzheimer’s disease (AD) — such as depression, anxiety, aggression, and apathy — and there’s a fascinating aspect to this that I’ll cover shortly.
But as great as this could be, we want more. We need something that can knock AD out, not temporarily diminish the signs of its destruction.
Fortunately, research is picking up, and new studies suggest classic psychedelics might prevent or treat AD and other neurodegenerative disorders [1].
There are multiple ways psychedelics could help — they tend to be connected, but here’s the thinking, in a nutshell. I’ll go over each aspect more in-depth.
(Remember, research is very limited and very new, so this is based on the little we know.)
- Inflammation is an underlying factor in Alzheimer’s.
- Depression is a risk factor for AD.
- SSRIs are anti-inflammatory and anti-depressants.
- Classic psychedelics work similarly to SSRIs and can help with the above.
- Classic psychedelics have other mechanisms of action that might help treat or prevent AD by stimulating neurogenesis and encouraging neuroplasticity, among other things.
Possible Contributions to AD & How Psychedelics Might Help
Sage Journals has the best explanation of Alzheimer’s, so I’ll just summarize it instead of trying to be creative [2].
“Amyloid-β (Aβ) plaques build up in the cortex and spread to subcortical areas. Tau neurofibrillary tangles begin in the entorhinal cortex, spread to the hippocampus, and end up in neocortical regions. These accumulate and cause a loss of neurons and synapses, resulting in dementia.”
Basically, Aβ plaques cause dendritic spines to degenerate rapidly, and tau silences the synapses [2].
The obvious question is what causes these amyloid plaques and tau tangles. After all, one-third of individuals over 80 are cognitively normal, even though they have about the same levels of Aβ and tau as those in the moderate stage of AD. (Hint: This has to do with cognitive resilience and reserve, and I’ll discuss that later.)
Despite our vast medical advances, there’s still a lot we don’t understand about the brain or Alzheimer’s — let alone psychedelics’ effects on either. What we do know is nothing short of fascinating and promising.
Let’s look at some of the possible causes of AD and how psychedelics could impact them.
A Quick Summary of Psychedelics, the Brain, & Alzheimer’s
Serotonergic psychedelics are called this because of how they interact with serotonin receptors, primarily 5-HT2A, but others as well.
However, they impact more than that, including the following systems [3]:
- Monoamines (dopamine, noradrenaline, octopamine, & serotonin)
- Glutamate
- Gamma-aminobutyric (GABA)
- Opioid system
- Inflammation & immune function
- Downstream systemic neural changes
Some research shows ketamine and serotonergic psychedelics’ downstream actions target GABA receptors and rapamycin complex 1 (mTORC1) signaling [4].
Because of these actions, SPs can stimulate neurogenesis and neuroplastic changes, significantly lowering neuroinflammation and making them good candidates for helping a range of psychiatric and neurological conditions — including dementia [1].
Add this to their high safety profile, and we could have a winning combination [5, 6].
For some, this is enough information to be mildly convinced, and they won’t feel the need to go further. That’s fine; my feelings aren’t hurt if you stop reading.
It’s like baking. You can follow a recipe and end up with a perfect loaf of bread without understanding how yeast works or why you have to knead the dough. But some people like to know the reason behind each step and what’s going on at a molecular level.
There’s a lot, and it all ties together, and I’m trying to understand and simplify it, so here goes. It might be messy.
Also, remember — this research is new and inconclusive. We’re at the tip of the iceberg with psychedelic research and Alzheimer’s.
1. Inflammation, AD, & Psychedelics
Neuroinflammation goes beyond being a pathological hallmark of Alzheimer’s. Every risk factor we know of — genetic and environmental — is associated with it [1]. It’s a big deal, which is why it’s being researched as a way to prevent AD.
Classic psychedelics’ affinity for 5HT2A-R gives them unique anti-inflammatory properties that target brain tissue. Specifically, they help control amyloid precursor (APP) processing loops — which I discuss next — and balance out chronic microglial activation [7].
Microglia are like the brain’s janitor; they’re in charge of cleaning out cellular waste, dead cells, neurons that don’t communicate properly, and proteins implicated in neurodegenerative disorders. However — they do this by causing inflammation, the body’s way of fighting infection. When they’re causing ongoing inflammation, as they do for Alzheimer’s, healthy brain cells start to die. Keeping them under control saves brain cells.
How funny that we’ve been taught drugs kill brain cells, and here we are discovering that they just might help protect them….
2. Amyloid-Beta
Interestingly enough, LSD (lysergic acid diethylamide) shows extra benefits — it easily crosses the blood-brain barrier (BBB), something common NSAIDs fail to do [1].
All serotonergic psychedelics modulate amyloid precursor protein (APP) processing loops and have potent anti-inflammatory effects.
However, studies show chronic use of LSD also reduced cortical soluble amyloid-beta (Aβ) proteins 40 and 42; both are isoforms of APP, but Aβ42 is thought to be particularly toxic.
Let me back up for a second. Amyloid-beta is what triggers Alzheimer’s progression and is a marker for the disease. These form neuritic plaques and neurofibrillary tangles and have been the main target for AD medications until recently [8].
It’s hypothesized that Aβ42 sets off the underlying issues of cognitive impairment. However, researchers haven’t broken down the changes in synaptic dysfunction that happen in the early stages of AD [9], so how or why they occur is still unknown.
3. Neuroplasticity, Neurogenesis, & Psychedelics
There’s strong evidence that SPs [10, 11, 12]:
- Increase neurogenesis
- Increase synapse number and function, likely due to their stimulating TrkB, mTOR, and 5-HT2A signaling pathways, causing structural changes
- Promote genes related to neuroplasticity, synaptic strength, dendritic growth
Neurogenesis is the process of new brain neurons forming. Decreased neuron production is linked to psychiatric and neurodegenerative disorders and can make cognitive deficits more pronounced.
Adult neurogenesis happens in two brain regions — the subgranular zone (SGZ) and the subventricular zone (SVZ) [13]. Anti-depressant drugs can reverse the effects of SGZ decline and enhance neurogenesis in the SVZ [14].
Neuroplasticity is summed up like this [15] — “Plasticity refers to the ability of the nervous system to dynamically modulate its function in response to ongoing internal activities or external experiences. Plasticity is a normal and essential part of cognition, also an important means by which the brain can respond to damage.”
It can break down even further. There’s synaptic plasticity, which is how well transmissions cross the synapses, and structural plasticity, which is the synapses’ physical structure.
It’s possible that the synapses are the link between amyloid and tau pathologies and the cognitive symptoms of AD. Boosting neuroplasticity could help improve cognition in AD patients.
Other research backs this up and takes it a step further, blaming it on dendritic spines.
Dendritic Spines
Dendric spine loss strongly correlates to cognitive impairment in Alzheimer’s and is yet another avenue researchers are looking at for treating this disease.
These spines are protrusions on the dendrites located in most of the excitatory synapses in the brain and play a critical role in synaptic functioning [2].
Here’s a rabbit trail. There are different classifications of the spines based on their shape — thin, stubby, and mushroom. Ironically, psilocybin, the main compound in magic mushrooms, increases spine density and size, essentially remodeling their structure and making changes that last at least one month. This comes along with increased neurotransmission.
Other SPs also seem to cause transient increases in spine size and the number of spine branches. Even an analog of ibogaine, which is not an SP, increased the spines [16].
Synapses
Reduced neurons and synaptic structural changes, abnormalities, damage, loss, and dysfunctions happen in the early stages of Alzheimer’s and other neurodegenerative diseases — often before any behavioral symptoms show [8, 17].
Late-stage AD goes hand-in-hand with an enormous loss of neurons and synapses, which is when the damage becomes apparent [15].
An article in Science Direct says psilocybin’s “synaptic rewiring in the cortex is fast and enduring,” and other SPs show similar results [16].
One hypothesis for why the effects last so long is because of their ability to cause neurons in the pre-frontal cortex (PFC) to grow, leading to structural and functional neuroplasticity and restored synaptic connections. Many psychiatric disorders show the atrophy of these neurons [18,19].
Another study showed that LSD, psilocybin, DMT, and DOI do the following [11]:
- Promote immediate early genes (IEGs) and brain-derived neurotrophic factor (BDNF) expressions, among others that have to do with synaptic plasticity
- Increase long-term potentiation
- Cause synaptic and dendritic growth
- Stimulate 5-HT2A receptors on GABAergic interneurons
Side notes:
IEGs have been observed in recognition and memories, and changes in their expression are associated with psychiatric disorders.
BDNF is a molecule involved with memory and learning. It’s highly regulated, and changes are associated with aging and psychiatric disease.
S1R Receptors
Here we have yet another target for AD treatment. There are two subtypes — sigma-1 and sigma-2.
Sigma 1 receptors (S1Rs) were considered a sub-type of opioid receptors, but now it’s accepted that they are not. Instead, they bind to many classes of psychotropic drugs.
Sigma-1 receptors are mostly in the laminae of the cortex, olfactory bulb, hypothalamus, mesencephalon, and Purkinje cells — parts that are for higher-brain functions like memory and drug dependence.
These receptors do many helpful things in regard to AD and the brain, such as [20, 21]:
- Prevent amyloid-B-induced neurotoxicity
- Are neuroprotective
- Encourage neuroplasticity
- Mobilize synaptic receptors
- Increase neuroimmunomodulation
- Control neurogenesis
On the other hand, losing S1Rs speeds up neurodegeneration; recessive mutations are linked to some forms of dementia, and polymorphisms increase the risk of AD [22].
DMT & S1Rs
A 2021 study [23] looked at N,N-Dimethyltryptamine (DMT) and PRE084 — both S1R agonists — and how they affected the brain.
DMT bound to S1R moderately and had a high affinity for 5-HT receptors. It significantly reduced astrogliosis — changes that happen to astrocytes due to CNS injury or disease. These cells have many roles — they provide nutrients, recycle neurotransmitters, and do other jobs that help keep things balanced.
DMT is also anti-inflammatory [24] and increases dopaminergic neurotransmission, along with synaptic plasticity and neurogenesis, but has other potential regarding memories. Anecdotal reports say ayahuasca helps retrieve memories; using DMT and MAOIs might activate memory-related parts of the brain and have anti-amnesic effects [20].
This alone has fascinating implications.
Psychedelics, Depression, & AD
Depression and Alzheimer’s are a little too comfortable with each other — their relationship is bidirectional, meaning depression is a risk factor for AD, and AD is a risk factor for depression.
In fact, it’s so bad that Alzheimer’s patients with symptoms of depression (about 50%) [25] rarely see positive results with typical treatment — multiple trials show SSRIs are ineffective.
In these cases, depression might be due to severe neurodegeneration and not actual clinical depression. Several factors can cause depression in AD patients — tau pathology, amyloid accumulation, inflammation, and faulty neurotransmitter signaling [26].
Other brain changes overlap, showing up in AD and late-life depression, like reduced hippocampal volume and neuronal death in areas related to emotions and cognition.
Could this be where psychedelics step in and save the day? Possibly — they are shown to be quite effective for depression.There could be multiple ways this could go; maybe psychedelics would treat depression brought on by AD, help prevent the disease in the first place, or both.

Psychedelics & Age-Related Issues (Benefits for Everyone!)
Time doesn’t discriminate, and we all get old. Here are some ways psychedelics might help your brain, even without AD.
Common Age-Related Issues
Age brings many issues that, coincidentally — or not — run parallel to many issues psychedelics can “fix” [27]:
- Less empathy
- Fewer meaningful experiences
- Existential anxiety
- Increased depression, psychological distress, and suicidality
- Reduced creativity, both divergent and convergent
- Reduced “openness” (the personality trait)
And while I can’t cover these in detail right now, there are plenty of articles if you’re interested in reading about how psychedelics can combat the above:
- Psychedelics for Creativity: Can Psychedelics Make Us More Creative?
- Medicine for the Mind
- Shrooms for Existential Anxiety & End-of-Life Care
- Can Psychedelics Treat Narcissism? Psychedelics & Empathy
It’s depressing to think about, but meaningful experiences tend to diminish with age. It doesn’t have to be that way, though — sometimes it’s a matter of perspective, and sometimes it means getting out more or finding your purpose [27].
And psychedelics can help with that.
In a 2006 study [28], two-thirds of the participants said their psychedelic experience was one of the top five most meaningful experiences of their lives.
Cognitive Resilience & Reserve
Cognitive resilience and reserve are similar but not the same, and while they might sound hokey, both are proving to be very real [29].
Cognitive resilience is remaining cognitively normal despite insults to the brain, including disease, stroke, traumatic brain injury, etc. [2] It has to do with how the brain compensates and pre-existing processing.
Cognitive reserve is something structural that some brains have that allows them to function normally even if they lose a lot of substrate.
As I mentioned earlier, many people have greater cognitive abilities than they should, considering their brain pathology. Physical and mental activity and genetics play a role in how well the brain handles adversity — notice that we have control over two out of three of those.
Can Psychedelics Treat the Symptoms of AD?
Neuropsychiatric symptoms (NPS) happen to 97% of AD patients, and 85% of them develop symptoms within five years of being diagnosed, with apathy and depression being the most common. NPSs cause rapid cognitive decline, greatly affect the quality of life — for the patient and the caregiver — and can be challenging to diagnose and treat [30].
Common neuropsychiatric symptoms of AD include:
- Abnormal motor behavior
- Agitation
- Anxiety
- Apathy
- Appetite and sleep problems
- Delusions
- Depression
- Disinhibition
- Elation
- Hallucinations
There are no FDA-approved medications for the symptoms of dementia. Any medications used — usually psychotropic drugs — are off-label and come with risks. Cannabinoids and psychedelics are the alternative options — much safer ones [31].
The serotonergic system is believed to cause 90% of the symptoms [32].
There’s little to no research on how psychedelics affect AD symptoms, but it would make sense that these substances could help, given their strong and long-lasting anxiolytic and anti-depressant effects.
Depression
As mentioned previously, depression and AD have a close relationship.
Psychedelics are already taking the world by storm for their anti-depressant effects. However, depression caused by AD is a different problem.
Time will tell if psychedelics can help in this case or not. If we were placing bets, I’d put my money on them.
This topic is extensive and beyond the scope of this article. If you’re curious about what the hype is about, read Medicine for the Mind or LSD for Depression.
Pain
Agitation is an intractable NPS that affects about 60% of AD patients and is associated with pain [30]. It makes sense. Of course someone will be agitated if they’re in pain all the time; it’s even worse if they have trouble communicating it.
And unfortunately, chronic pain is another mechanism we don’t fully understand.
We do know that experiencing pain over an extended period causes the neural circuitry to be strengthened.
Research, again, is limited but shows psychedelics have potent analgesic effects for pain from cancer, phantom limbs, and cluster headaches [33].
How, exactly, psychedelics would treat chronic pain is unknown, but here are a few of the possibilities:
- There are similarities between 5-HT2A activation pathways and nociceptive modulation pathways.
- Psychedelics can reset areas of functional connectivity that play a role in neuropathic states, possibly reversing the changes in neuronal connections in chronic pain states.
Given their safety profile, this is certainly an option worth pursuing. Maybe this could help kill two birds with one stone — knock out agitation and pain.
Impulse Control
Psilocybin could help with an AD patient’s impulse control by helping to improve the limbic system’s functioning. This system is involved with memory and emotions [1].

Psychedelic Use In the Elderly: Is It Safe?
As you can imagine, there’s not much information on how psychedelics affect the elderly. No one is dragging their grandmother in for clinical testing on MDMA, and the older crowd is not documenting their microdosing experiences on Reddit.
One 2019 study had 48 healthy volunteers take LSD for 21 days (I never said they were nonexistent) — the mean age was 62 [7]. They were divided into groups of twelve, and each group received either 5, 10, or 20 micrograms (μg) or a placebo.
Researchers checked the volunteers’ cognition, balance, and proprioception and found no impairment. Other safety assessments were done as well, including blood pressure, pulse rate, clinical laboratory evaluations (i.e., hematology, blood chemistry, urinalysis), electrocardiogram (ECG) parameters, and physical examinations.
All assessments determined that low doses of LSD were well-tolerated and carried no risk. Mild to moderate headaches were the biggest complaint.
Cardiovascular Disease
One possible issue is the potential for cardiovascular adverse events, either indirectly or directly.
Considering cardiovascular disease is the leading cause of death in America (2023 statistics), this is something that impacts more than the elderly.
5-HT2A has vasoconstriction effects and is implicated in an increase in vascular smooth muscle cells, along with their migration. This mechanism could be a part of the development of atherosclerosis — or the buildup of plaque in the artery walls.
Even healthy volunteers consistently show an increase in heart rate and blood pressure when given LSD, DMT, and psilocybin.
Due to the possible risks, any history of cardiovascular disease or anomalies is enough of a reason to be excluded from clinical trials [34].
Related: Can Psychedelics Increase the Risk of Heart Disease?
General Side Effects That Apply to Everyone
The side effects vary, depending on the substance. It’s best to learn as much as you can about any drug before using it — this applies to illegal, prescription, or over-the-counter. Everything has adverse effects, especially when used improperly or with other drugs.
That said, these psychedelics are proven to be quite safe and well-tolerated by most people [5, 35].
Common side effects include:
- Anxiety
- Headaches
- Nausea
Fatigue, low mood, and anxiety occurred as late adverse events, mostly with MDMA.
Microdosing provides the least risk because the amount used is so small.
If you’re using larger amounts, read our guide (below) first so your trip is as safe as possible.
Start with the following articles if you’re new. Actually, read these anyway. You might be surprised at some of the information.
- Magic Mushrooms 101
- LSD (Acid): The Ultimate Guide
- MDMA (Ecstasy): Benefits, Research, & Safety
- What Is DMT?
- The Ultimate Guide to Microdosing Psychedelics
- Tripsitter’s Guide to Safe Tripping
One “side effect” — possibly one of the most dangerous, ironically — is the legal ramifications. Check your local laws and know the risks before you do something that could destroy your life.
What Is Alzheimer’s Disease?
I covered the more scientific explanation of Alzheimer’s earlier, so here’s a more basic version.
According to the Alzheimer’s Association’s 2023 data, about 1 in 9 (10.8%) adults over 65 have Alzheimer’s. The estimated lifetime risk at age 45 is 1 in 5 for women and 1 in 10 for men, and the risk increases with age.
Alzheimer’s is a neurodegenerative disease — and a global health threat, according to the World Health Organization [36]. These diseases cause nerve cells in the brain to lose function and eventually die, impacting most activities, including a person’s balance, talking, breathing, and heart function.
AD usually starts in the parts of the brain that have to do with memory, but, like most diseases, it can’t be too predictable. Atypical Alzheimer’s begins in other parts and produces completely different symptoms. Posterior cortical atrophy is one example — it starts in the back of the brain and causes vision problems.
Genetics, medical conditions, tumors, strokes, toxins, and viruses can all be blamed for nerve disorders.
So far, there is no treatment, and we can’t even slow its progression. All we can do is hope to relieve some of the symptoms for a little while.
Other neurodegenerative nerve diseases include:
- Amyotrophic lateral sclerosis
- Friedreich ataxia
- Huntington’s disease
- Lewy body disease
- Parkinson’s disease
- Spinal muscular atrophy
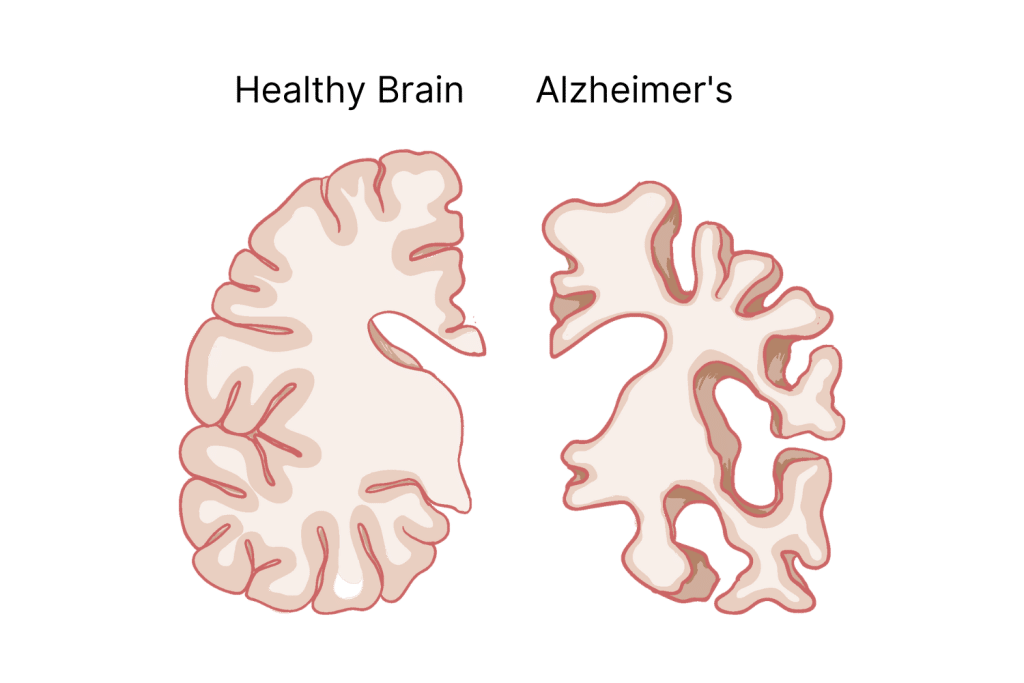
What’s the Difference Between Alzheimer’s & Dementia?
Dementia is a term used for any loss of memory, communication, behavior, and cognitive ability — such as problem-solving skills — that interferes with a person’s life.
Alzheimer’s causes so much damage that it causes dementia. In fact, AD is the most common cause of it.
Symptoms of AD
Frighteningly enough, plaques and tau tangles can form long before the symptoms show up. Although, this could be a helpful thing if we manage to find ways to fix the problem before the damage is done.
According to the National Institute on Aging, here’s what you can expect to see once the problems decide to rear their ugly head.
Mild (Early-Stage) Symptoms of AD
- Aggression
- Anxiety
- Daily tasks take longer than usual
- Difficulty completing tasks
- Forgetting new information
- Forgetting the date
- Has a hard time planning or solving problems
- Less spontaneous
- Losing things or misplacing them in odd places
- Memory loss
- Mood and personality changes
- Not knowing the location
- Poor judgment
- Repeating questions
- Trouble with money and bills
- Unmotivated
- Wandering and getting lost
Moderate (Mid-Stage) Symptoms of AD
- Changes in sleeping patterns
- Difficulty organizing thoughts and thinking logically
- Difficulty with language, reading, writing, and working with numbers
- Emotional outbursts
- Hallucinations, delusions, and paranoia
- Has trouble learning new things
- Impulsive behavior
- Inability to do familiar tasks
- Increased confusion and memory loss, including personal history
- Less social
- Problems recognizing family and friends
- Repetitive statements, movements, and muscle twitches
- Restlessness, agitation, anxiety, wandering
- Shortened attention span
- Stressed by new situations
Severe (Late-Stage) Symptoms of AD
- Difficulty swallowing
- General physical decline
- Groaning, moaning, or grunting
- Increased sleeping
- Loss of bowel and bladder control
- No interest in eating
- Seizures
- Unable to communicate
- Unaware of recent experiences or surroundings
- Weight loss
Current Treatments for Alzheimer’s Disease
There isn’t anything that can prevent or cure Alzheimer’s, but current treatments help reduce or control the cognitive and behavioral symptoms, temporarily.
AChE Inhibitors
Acetylcholinesterase (AChE) inhibitors, also called cholinesterase inhibitors, help by preventing acetylcholine — a chemical important for memory and thinking — from breaking down. These are prescribed for mild to moderate symptoms and become less effective as the disease progresses. Another issue with their effectiveness is dosage limitations due to the side effects [37].
Common AChE inhibitors include:
- Donepezil
- Galantamine
- Rivastigmine
- Tacrine
Common adverse side effects include:
- Abnormal dreams
- Aggression
- Agitation
- Anorexia
- Constricted airways
- Constriction of the pupils
- Diarrhea
- Dizziness
- Fainting
- Fatigue
- Hallucinations
- Headache
- Increased secretion of sweat, saliva, and tears
- Insomnia
- Itching
- Mucus secretion in the respiratory tract
- Muscle cramps
- Nausea
- Pisa syndrome
- Rash
- Slow heart rate
- Urinary incontinence
- Vasodilation
- Vomiting
N-methyl-D-aspartate (NMDA) Antagonist
Memantine is an NMDA antagonist and is thought to regulate glutamate, which can cause brain cells to die in excessive amounts. It’s prescribed in the later stages of AD and helps a person manage daily functions a little longer. It can be used with AChE inhibitors.
Possible side effects of memantine include:
- Aggression
- Confusion
- Constipation
- Cough
- Depression
- Diarrhea
- Dizziness
- Hallucination
- Headache
- Nausea
- Pain, especially in the back
- Shortness of breath
- Sleepiness
- Vomiting
- Weight gain
The FDA has approved rivastigmine, a patch combining memantine and donepezil, for treating moderate to severe AD.
Reducing the Risk for Alzheimer’s
The situation might sound dire, and in a sense, it is. But we aren’t entirely helpless. We can take control of certain areas of our lives that reduce our chances of developing Alzheimer’s and about every other disease.
Even better, these steps are almost too good to be true — they’re relatively inexpensive and easy, especially considering the steep price of not taking them.
Chart: Factors Affecting Alzheimer’s Disease:
| RISK FACTORS | PROTECTIVE FACTORS |
| Type 2 Diabetes | Higher Education/ Continued Learning |
| Large Variability In Blood Pressure | Cognitive Activity |
| Dyslipidemia | Bilingualism |
| Midlife Obesity | Social Engagement |
| Cardiovascular Diseases | Marriage |
| Traumatic Brain Injury | Physical Activity |
| Hyperhomocysteinemia | Moderate Alcohol Consumption |
| Hearing Loss | Moderate Coffee and Tea Consumption |
| Oral Disease | Mediterranean Diet |
| Depression and Stress | MIND Diet |
| Sleep Disturbances | Ketogenic Diet |
| Smoking | Vitamins and Flavonoids |
| Air Pollution | Fish Intake |
| Anticholinergic Medication | HDL-Cholesterol |
Chart from The Epidemiology of Alzheimer’s (2021) [38]
Diet
A nutritional diet is one of the most extensively studied ways of slowing Alzheimer’s progression, and it’s two-fold. Nutritional elements directly influence AD’s development and severity, and on the flip side, metabolic disorders from an unhealthy diet are a huge cause of it.
In other words, eating healthy can help prevent Alzheimer’s, but eating unhealthy can actually cause it.
In fact, researchers are looking at how to use flavonoids, terpenoids, and alkaloids as potential ways to treat AD [39].
Nanoscience might help them find new ways to give the central nervous system more direct exposure to these neuroprotective agents.
Here are a few examples of these highly potent bioactive compounds that could help [40].
Beneficial Foods for AD
- Chalcones — These are a subgroup of open-chain flavonoids and are found in tomatoes and ladies’ fingers. Researchers are studying them due to their many biological effects that could help ward off Alzheimer’s.
- Curcumin — This is the active ingredient in turmeric and has many biological activities, including anti-inflammatory, antioxidant, and neuroprotective.
- Flavanones — You’ll find an abundance of these in citrus fruits like lemons and oranges. They are anti-inflammatory, lower blood lipids, and scavenge free radicals.
- Flavones — These are in many medicinal plants and have antioxidant, anti-inflammatory, and neuroprotective capabilities.
- Flavonoids: Fruits and vegetables have large amounts of these. They are neuroprotective and scavenge free radicals, helping reduce the neuroinflammation in Alzheimer’s.
- Isoflavones — Leguminous plants like soybeans have an abundance of these compounds that have inhibitory actions on AChE and MAOB.
- Resveratrol — This non-flavonoid is in foods like red wine, almonds, and grapes and is anti-inflammatory, anti-carcinogenic, neuroprotective, and antioxidant.
The Mediterranean and MIND diets can be one of the best ways to get the nutrients your brain needs [39].
The Mediterranean diet (MeDi) consists of foods that provide all the essential nutrients. Studies show it is highly neuroprotective and could be a way to prevent cognitive decline and impairment.
The MIND diet‘s full name is “Mediterranean-DASH Intervention for Neurodegenerative Delay,” and based on foods that studies suggest help prevent dementia. How can you go wrong with a diet named that?
Exercise
Yes, of course, exercise is on this list too. Not only should you watch what you eat, but you should also move more. I guarantee you’ll feel better overall.
Need more motivation?
Daily physical activity was related to a half-decreased risk of Alzheimer’s. It also helps keep other nasty diseases at bay, like cardiovascular conditions, diabetes, chronic respiratory diseases, and even some cancers.
Not only that — exercise has cognitive benefits that can delay neurodegeneration. Aerobic exercise specifically benefits executive function and overall neuronal health.
There are many ways exercise helps the body that, in turn, specifically helps prevent Alzheimer’s [38, 41].
Here are a few of those ways, for those that care:
- Increasing exercise-induced metabolic factors and muscle-derived myokines promotes neurogenesis, which stimulates the production of neurotrophins such as BDNF.
- Exercise improves the brain redox status, which also helps Aβ deposition and other hallmarks of AD.
Social Life & Human Connection
Finally, something everyone can get behind. Well, almost everyone. Some people (me) prefer to avoid most social things.
But it’s time to get over it. Frequent social contact can moderately reduce the risk of dementia and is beneficial for cognitive function in general.
Now you have an excuse to go out with friends. Or don’t have a good reason to stay home. Depends on how you look at it.
Do it for your brain’s health.
Continue to Learn
Learning another language is apparently a great way to build cognitive reserve.
If you aren’t up for that, do anything that makes your brain work and exposes you to new things. Read a book, learn a new skill, play cognitive games (on your phone is fine), travel, eat weird foods, or listen to a variety of music genres.
Studies show that learning can decrease amyloid burden and attendant neuroanatomical and behavioral deficits [42].
Your brain isn’t really a muscle, but pretend it is. You can actually build brain volume along with improving brain functioning. Actively engaging your mind can reduce the risk of Alzheimer’s by 46% — not too bad, considering you can do this one from the couch.
It’s important to note that lifestyle changes probably won’t be enough to prevent Alzheimer’s from developing, especially if the changes are made close to or after clinical onset. It takes years of exposure for these lifestyle factors to have the chance to delay AD’s onset or progression [42].
Living a healthy life benefits you in many ways, though, so it never hurts to try.

Future Direction of AD Treatment (Besides Psychedelics)
There are a few surprising ways that we might get a leg up on AD beyond treating it with medications.
Mild Behavior Impairment
Mild behavior impairment is a possible symptom that shows up before the clinical symptoms of dementia. It could be another predictor of the disease, and early intervention might help prevent cognitive decline [43].
Iron
An increasing amount of evidence suggests iron levels in the brain play a role in Alzheimer’s. A special imaging probe showed increased iron redox in the same areas with Aβ plaques. This information can provide new insights into the causes of AD and how to treat it [44].
Sleep Medications
Sleep disturbances — such as having trouble falling or staying asleep — can be another early sign of Alzheimer’s. It becomes a vicious cycle as AD causes changes in the brain that interrupt sleep, and poor sleep quality speeds up cognitive decline.
One small study showed a drop in Alzheimer’s proteins in individuals that took a sleeping pill — suvorexant, to be exact — before bed.
This was a very small study, however, but it shows the possibility that sleep medications might help slow AD progression [45]. However, the researchers do not recommend taking nightly sleeping pills to ward off AD.
New Medications for Alzheimer’s
As far as medication or treatment goes, they will be more effective in the early stages, before much neurodegeneration has occurred and the damage is done [36].
Researchers are targeting the synapses, hoping to prevent, slow, or stop synaptic loss or strengthen the connections or density [8]. Other areas of focus are inflammation, the heart, insulin resistance, tau tangles, and hormones.
The following medications are currently in clinical trials.
Monoclonal Antibodies
Aducanumab and lecanemab received Accelerated Approval from the Food and Drug Administration (FDA).
Both are monoclonal antibodies selective for Aβ and are supposed to remove the amyloid plaques. However, there’s no evidence that clearing these plaques means less cognitive decline.
While this sounds like progress, aducanumab’s approval has caused strong debate in the medical community — you can read about it on the Duke Health, AAFP, and BMJ websites. There are two major problems with it — data from two Phase 3 trials were insufficient and did not prove its efficacy, and the drug comes with serious side effects [46]. In February 2023, the FDA made major changes to aducanumab’s label.
Aducanumab’s side effects include:
- Amyloid-related imaging abnormalities (ARIA)
- Amyloid-related imaging abnormalities due to hemosiderin deposition (ARIA-H) microhemorrhage
- ARIA-H superficial siderosis
- Confusion, delirium
- Diarrhea
- Difficulty breathing
- Disoriented
- Dizziness
- Edema
- Falling
- Headache
- Hives
- Itching
- Nausea
- Rash
- Small spots of bleeding on the brain
- Swelling of the brain
- Swelling of your face, lips, tongue, or throat
- Vision changes
Lecanemab is similar to aducanumab but causes cognitive decline to slow and has a slightly better safety profile.
Alzheimer’s Disease Research Center has information on any current research if you want to stay up-to-date.

A Little History Lesson, Just Because
We owe a lot to the people that came before us, whether it’s the indigenous groups that passed on their knowledge of the land and all it offers or those who dedicate their lives to figuring out how things work.
In this case, we’ll look at Alois Alzheimer, a German physician who had a knack for linking a patient’s symptoms to microscopic brain changes.
One patient of his — Auguste D. — suffered from memory loss despite not being elderly. Alzheimer noticed during the autopsy that the cerebral cortex was thin, and senile plaque and neurofibrillary tangles were around nerve cells.
Plaque wasn’t new to the medical field then but was typically only seen in older individuals. The tangles, however, hadn’t been described before.
The pathological diagnosis of AD is still based on these traits. This has remained essentially unchanged since 1906, when Alzheimer made his discovery, proving how monumental it really was.
Final Thoughts: Psychedelics & the Future of AD
There’s a pretty simple conclusion to this. Psychedelics are proving to be very safe when used responsibly. They make positive changes to the brain that could help treat or maybe even cure Alzheimer’s. Unfortunately, we won’t know until we try, and we can’t put effort into it until significant changes are made, both as a society and legally.
So far, we have made almost no progress on Alzheimer’s prevention, and what we do have isn’t very safe or effective. It’s time to shift gears and try something that’s been in front of us all along.
Subscribe For More Psychedelic Insight Straight to Your Inbox 🍄
References
- Vann Jones, S. A., & O’Kelly, A. (2020). Psychedelics as a treatment for Alzheimer’s disease dementia. Frontiers in synaptic neuroscience, 12, 34.
- Walker, C. K., & Herskowitz, J. H. (2021). Dendritic spines: Mediators of cognitive resilience in aging and Alzheimer’s disease. The Neuroscientist, 27(5), 487-505.
- Nagra, J. Psychedelic Agents as Potential Therapeutics for Obsessive-Compulsive Disorder.
- Johnston, J. N., Kadriu, B., Allen, J., Gilbert, J. R., Henter, I. D., & Zarate Jr, C. A. (2023). Ketamine and serotonergic psychedelics: An update on the mechanisms and biosignatures underlying rapid-acting anti-depressant treatment. Neuropharmacology, 109422.
- Andersen, K. A., Carhart‐Harris, R., Nutt, D. J., & Erritzoe, D. (2021). Therapeutic effects of classic serotonergic psychedelics: A systematic review of modern‐era clinical studies. Acta Psychiatrica Scandinavica, 143(2), 101-118.
- Schlag, A. K., Aday, J., Salam, I., Neill, J. C., & Nutt, D. J. (2022). Adverse effects of psychedelics: From anecdotes and misinformation to systematic science. Journal of Psychopharmacology, 36(3), 258-272.
- Family, N., Maillet, E. L., Williams, L. T., Krediet, E., Carhart-Harris, R. L., Williams, T. M., … & Raz, S. (2020). Safety, tolerability, pharmacokinetics, and pharmacodynamics of low dose lysergic acid diethylamide (LSD) in healthy older volunteers. Psychopharmacology, 237, 841-853.
- Peng, L., Bestard-Lorigados, I., & Song, W. (2022). The synapse as a treatment avenue for Alzheimer’s Disease. Molecular Psychiatry, 27(7), 2940-2949.
- Andrade-Talavera, Y., & Rodríguez-Moreno, A. (2021). Synaptic plasticity and oscillations in Alzheimer’s disease: A complex picture of a multifaceted disease. Frontiers in Molecular Neuroscience, 14, 696476.
- Ly, C., Greb, A. C., Cameron, L. P., Wong, J. M., Barragan, E. V., Wilson, P. C., … & Olson, D. E. (2018). Psychedelics promote structural and functional neural plasticity. Cell reports, 23(11), 3170-3182.
- Calder, A. E., & Hasler, G. (2023). Towards an understanding of psychedelic-induced neuroplasticity. Neuropsychopharmacology, 48(1), 104-112.
- Khan, S. M., Carter, G. T., Aggarwal, S. K., & Holland, J. (2021). Psychedelics for brain injury: a mini-review. Frontiers in Neurology, 12, 685085.
- Ming, G. L., & Song, H. (2011). Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron, 70(4), 687-702.
- Apple, D. M., Fonseca, R. S., & Kokovay, E. (2017). The role of adult neurogenesis in psychiatric and cognitive disorders. Brain Research, 1655, 270-276.
- Jackson, J., Jambrina, E., Li, J., Marston, H., Menzies, F., Phillips, K., & Gilmour, G. (2019). Targeting the synapse in Alzheimer’s disease. Frontiers in neuroscience, 13, 735.
- Shao, L. X., Liao, C., Gregg, I., Davoudian, P. A., Savalia, N. K., Delagarza, K., & Kwan, A. C. (2021). Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo. Neuron, 109(16), 2535-2544.
- Koch, G., & Spampinato, D. (2022). Alzheimer disease and neuroplasticity. Handbook of Clinical Neurology, 184, 473-479.
- Olson, D. E. (2022). Biochemical mechanisms underlying psychedelic-induced neuroplasticity. Biochemistry, 61(3), 127-136.
- Grieco, S. F., Castrén, E., Knudsen, G. M., Kwan, A. C., Olson, D. E., Zuo, Y., … & Xu, X. (2022). Psychedelics and Neural Plasticity: Therapeutic Implications. Journal of Neuroscience, 42(45), 8439-8449.
- Inserra, A. (2018). Hypothesis: the psychedelic ayahuasca heals traumatic memories via a sigma 1 receptor-mediated epigenetic-mnemonic process. Frontiers in pharmacology, 9, 330.
- Hayashi, T., & Su, T. P. (2005). The sigma receptor: evolution of the concept in neuropsychopharmacology. Current neuropharmacology, 3(4), 267-280.
- Ryskamp, D. A., Korban, S., Zhemkov, V., Kraskovskaya, N., & Bezprozvanny, I. (2019). Neuronal sigma-1 receptors: signaling functions and protective roles in neurodegenerative diseases. Frontiers in Neuroscience, 13, 862.
- Borbély, E., Varga, V., Szögi, T., Schuster, I., Bozsó, Z., Penke, B., & Fülöp, L. (2022). Impact of Two Neuronal Sigma-1 Receptor Modulators, PRE084 and DMT, on Neurogenesis and Neuroinflammation in an Aβ1–42-Injected, Wild-Type Mouse Model of AD. International Journal of Molecular Sciences, 23(5), 2514.
- Szabo, A., Kovacs, A., Frecska, E., & Rajnavolgyi, E. (2014). Psychedelic N, N-dimethyltryptamine and 5-methoxy-N, N-dimethyltryptamine modulate innate and adaptive inflammatory responses through the sigma-1 receptor of human monocyte-derived dendritic cells. PloS one, 9(8), e106533.
- Bianco, O. A. F. M., Manzine, P. R., Nascimento, C. M. C., Vale, F. A. C., Pavarini, S. C. I., & Cominetti, M. R. (2016). Serotoninergic anti-depressants positively affect platelet ADAM10 expression in patients with Alzheimer’s disease. International Psychogeriatrics, 28(6), 939-944.
- Elsworthy, R. J., & Aldred, S. (2019). Depression in Alzheimer’s disease: an alternative role for selective serotonin reuptake inhibitors?. Journal of Alzheimer’s Disease, 69(3), 651-661.
- Aday, J. S., Bloesch, E. K., & Davoli, C. C. (2020). Can psychedelic drugs attenuate age-related changes in cognition and affect?. Journal of Cognitive Enhancement, 4, 219-227.
- Griffiths, R. R., Richards, W. A., McCann, U., & Jesse, R. (2006). Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology, 187, 268-283.
- Jellinger, K. A., & Attems, J. (2022). Neuropathological approaches to cerebral aging and neuroplasticity. Dialogues in clinical neuroscience.
- Scuteri, D., Tonin, P., Corasaniti, M. T., & Bagetta, G. (2022). Targeting Neuroplasticity for the Management of Pain and Agitation in Alzheimer’s Disease via Bergamot Nanotherapy. OBM Neurobiology, 6(3), 1-11.
- Forester, B., Lanctôt, K., Mintzer, J., & Rosenberg, P. (2022). Cannabinoids and Psychedelics for Neuropsychiatric Symptoms of Alzheimer’s: Addressing Disparities Through Clinical Trials. The American Journal of Geriatric Psychiatry, 30(4), S7.
- Sarangi, A., & Akinkunmi, O. (2023). Psychedelics for the Neuropsychiatric Symptoms of Alzheimer’s disease. The American Journal of Geriatric Psychiatry, 31(3), S44-S45.
- Castellanos, J. P., Woolley, C., Bruno, K. A., Zeidan, F., Halberstadt, A., & Furnish, T. (2020). Chronic pain and psychedelics: a review and proposed mechanism of action. Regional Anesthesia & Pain Medicine, 45(7), 486-494.
- Neill, J. C., Shahid, M., Gittins, R., Schlag, A. K., & Tarazi, F. I. (2023). Risks and adverse events associated with the use of psychedelics. Psychedelics as Psychiatric Medications, 113.
- Schlag, A. K., Aday, J., Salam, I., Neill, J. C., & Nutt, D. J. (2022). Adverse effects of psychedelics: From anecdotes and misinformation to systematic science. Journal of Psychopharmacology, 36(3), 258-272.
- Mahaman, Y. A. R., Embaye, K. S., Huang, F., Li, L., Zhu, F., Wang, J. Z., … & Wang, X. (2022). Biomarkers used in Alzheimer’s disease diagnosis, treatment, and prevention. Ageing research reviews, 74, 101544.
- Arjona, A. A., Pooler, A. M., Lee, R. K., & Wurtman, R. J. (2002). Effect of a 5-HT2C serotonin agonist, dexnorfenfluramine, on amyloid precursor protein metabolism in guinea pigs. Brain research, 951(1), 135-140.
- Zhang, X. X., Tian, Y., Wang, Z. T., Ma, Y. H., Tan, L., & Yu, J. T. (2021). The epidemiology of Alzheimer’s disease modifiable risk factors and prevention. The journal of prevention of Alzheimer’s disease, 8, 313-321.
- Chauhan, P. S., Yadav, D., & Arukha, A. P. (2022). Dietary Nutrients and Prevention of Alzheimer’s disease. CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders), 21(3), 217-227.
- Islam, F., Khadija, J. F., Harun-Or-Rashid, M., Rahaman, M., Nafady, M. H., Islam, M., … & Mubarak, M. S. (2022). Bioactive compounds and their derivatives: an insight into prospective phytotherapeutic approach against alzheimer’s disease. Oxidative Medicine and Cellular Longevity, 2022.
- Valenzuela, P. L., Castillo-Garcia, A., Morales, J. S., de la Villa, P., Hampel, H., Emanuele, E., … & Lucia, A. (2020). Exercise benefits on Alzheimer’s disease: State-of-the-science. Ageing research reviews, 62, 101108.
- Selkoe, D. J. (2012). Preventing Alzheimer’s disease. science, 337(6101), 1488-1492.
- Jiang, F., Cheng, C., Huang, J., Chen, Q., & Le, W. (2022). Mild Behavioral Impairment: An Early Sign and Predictor of Alzheimer’s Disease Dementia. Current Alzheimer Research, 19(6), 407-419.
- Nikseresht, S., Bush, A. I., & Ayton, S. (2019). Treating Alzheimer’s disease by targeting iron. British journal of pharmacology, 176(18), 3622-3635.
- Lucey, B. P., Liu, H., Toedebusch, C. D., Freund, D., Redrick, T., Chahin, S. L., … & Bateman, R. J. (2023). Suvorexant acutely decreases tau phosphorylation and Aβ in the human CNS. Annals of neurology.
- Vaz, M., Silva, V., Monteiro, C., & Silvestre, S. (2022). Role of aducanumab in the treatment of Alzheimer’s disease: Challenges and Opportunities. Clinical Interventions in Aging, 797-810.