How Does Ketamine Work? Exploring Ketamine’s Mechanism of Action
Ketamine’s mechanism of action (MOA) is complicated. Its primary actions involve blocking glutamate binding sites (NMDA) — but this is just the tip of the iceberg.
Ketamine is a bizarre drug. It’s classified as a dissociative anesthetic — which means it induces a powerful “decoupling” of one’s physical body from one’s conscious experience.
Users feel as though they’re transported to another place outside their own body where they don’t feel pain and are often detached from their own emotions. In high doses, they lose touch with the environment around them and often “forget” who or what they are.
All of these effects, as well as an absence of autonomic inhibition (which controls our breathing), make ketamine an ideal anesthetic agent during surgical procedures. Doctors can anesthetize patients without worrying about asphyxiation or cardiac complications the way drugs like propofol, sufentanil, or remifentanil are known for. On top of these benefits, the effects of ketamine wear off completely in a matter of hours.
These qualities also make ketamine an attractive experience for psychonauts looking for out-of-body experiences.
What’s even more impressive is that recent studies involving ketamine have demonstrated a rapid antidepressant action following administration. This effect lasts between 1–2 weeks before fading away.
So, how does ketamine work? What are its target receptors, and how do these interactions provide powerful dissociative, antidepressant, anesthetic, and neuroplastic effects?
Related Guides: Ketamine 101 | Ketamine Therapy 101 | Signs of Ketamine Overdose
Summary: Ketamine Targets
| Target Receptor | Effect | Implications |
| NMDA (Glutamate) | Direct Inhibition (Strong) ⬇️ | Dissociation, Anesthesia |
| AMPA (Glutamate) | Indirect Activation ⬆️ | Neuroplasticity, Antidepressant |
| GABA | Potentiation ⬆️ | Sedative, Relaxant, Intoxicant |
| Opioid (mu-, delta-, kappa) | Direct Activation ⬆️ | Analgesic, Euphoric, Psychedelic (kappa-opioid) |
| Sigma 1 & 2 | Indirect Modulation | Psychomimetic, Neuroprotective, Antidepressant |
| Muscarinic (Cholinergic) | Direct Inhibition ⬇️ | Sedation, Increased Heart Rate, Hallucinations |
| Nicotinic (Cholinergic) | Direct Inhibition ⬇️ | Muscle Relaxation, Sedation |
| Monoamines (Dopamine, Serotonin, Norepinephrine) | Reuptake Inhibition ⬇️ | Mixed Effects, Antidepressant, Euphoric, Analgesic |
| Sodium Channels | Direct Inhibition ⬇️ | Anesthesia, amnesia, dissociation |
| Potassium Channels | Direct Inhibition ⬇️ | Anesthesia |
| mTOR | Direct Activation ⬆️ | Neuroplasticity, Antidepressant |
Become an Expert in Psychedelics by Subscribing to Our Newsletter
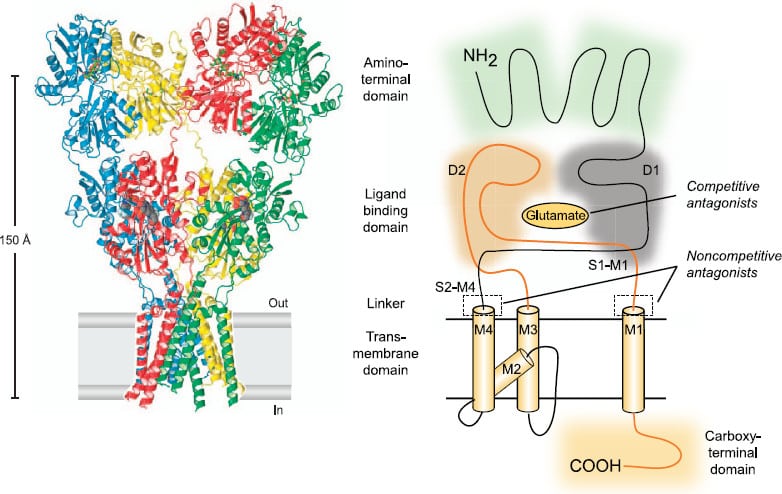
Glutamate Receptors (Ketamine’s Primary Mechanism of Action)
Glutamate is the most prevalent neurotransmitter in the central nervous system. It’s involved with learning, memory formation, and synaptic plasticity (the ability for neurons to form connections with other neurons).
Ketamine’s interaction with this neurotransmitter is considered the primary mechanism of action — primarily through the NMDA receptors [19].
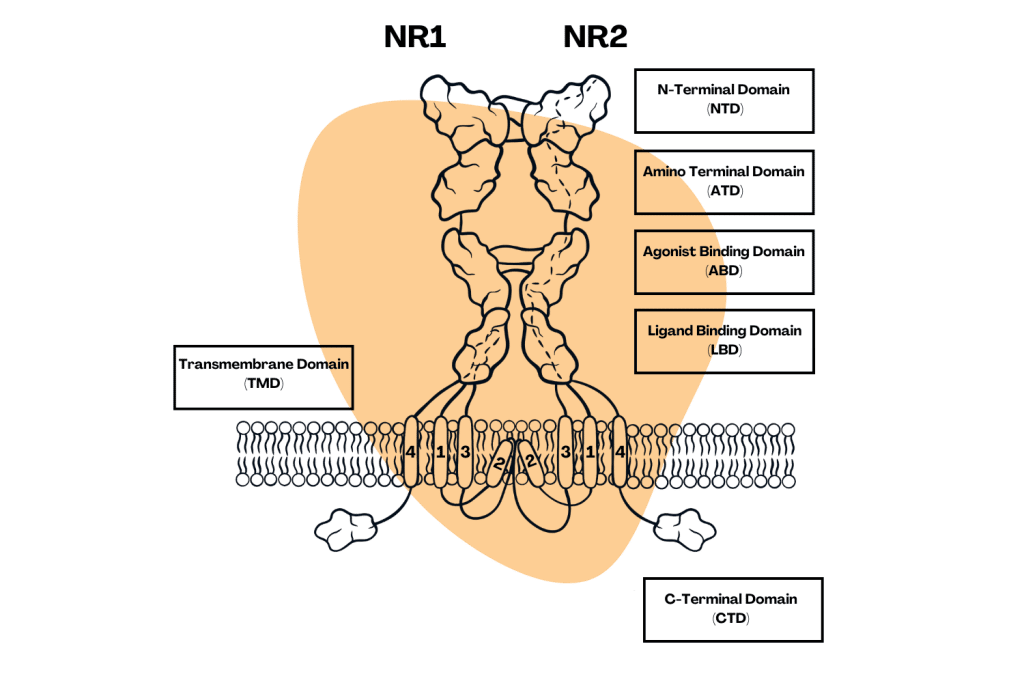
There are three main glutamate receptors in the brain — the NMDA (N‐methyl‐D‐aspartate), AMPA (alpha‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazole‐propionic acid), and KA (kainate) receptors. Both NMDA and AMPA are heavily involved in neuronal signaling and synaptic transmission, and both are believed to be primary targets of either ketamine or its metabolites, norketamine and hydroxynorketamine.
NMDA Receptors
The NMDA (N-methyl-D-aspartate) receptors are considered the primary mechanism for the dissociative, amnesic, psychosensory, and neuroprotective effects of ketamine [1].
Ketamine works by preventing glutamate from binding to the NMDA receptors. When this happens, sensory signals traveling to cortical regions of the brain are received but are not interpreted. This means things like sight, touch, sound, and pain signals reach the brain, but the brain is unable to interpret or make sense of the data and is subsequently lost. This effect, possibly combined with ketamine’s impact on the kappa-opioid receptors, sodium channels, and norepinephrine, is believed to be responsible for the drug’s distinct and bizarre psychoactive and dissociative qualities.
Some of the trickle-down effects of NMDA inhibition is the prevention or reduction of a condition called the “wind-up phenomenon,” which is characterized by increased pain sensitivity and chronic pain states such as fibromyalgia, migraine headaches, complex regional pain syndrome (CRPS), and more [2].
AMPA Receptors
Effect of ketamine & ketamine metabolites on AMPA: Agonistic (increased activity).
Ketamine is believed to indirectly modulate the AMPA receptors — which, like NMDA, is a type of glutamate receptor located in the central nervous system. Like NMDA, AMPA receptors play a role in synaptic transmission, plasticity, and learning and memory processes.
Ketamine itself likely has only a small impact on AMPA directly, but some studies suggest metabolic byproducts, including norketamine and hydroxynorketamine, may provide more substantial activation of these key glutamate receptors [3]. It’s also thought that ketamine may upregulate AMPA through indirect mechanisms that have not yet been discovered [4].
AMPA activation is thought to trigger the release of brain-derived neurotrophic factor (BDNF), which plays a key role in neuroplasticity in the brain [5].
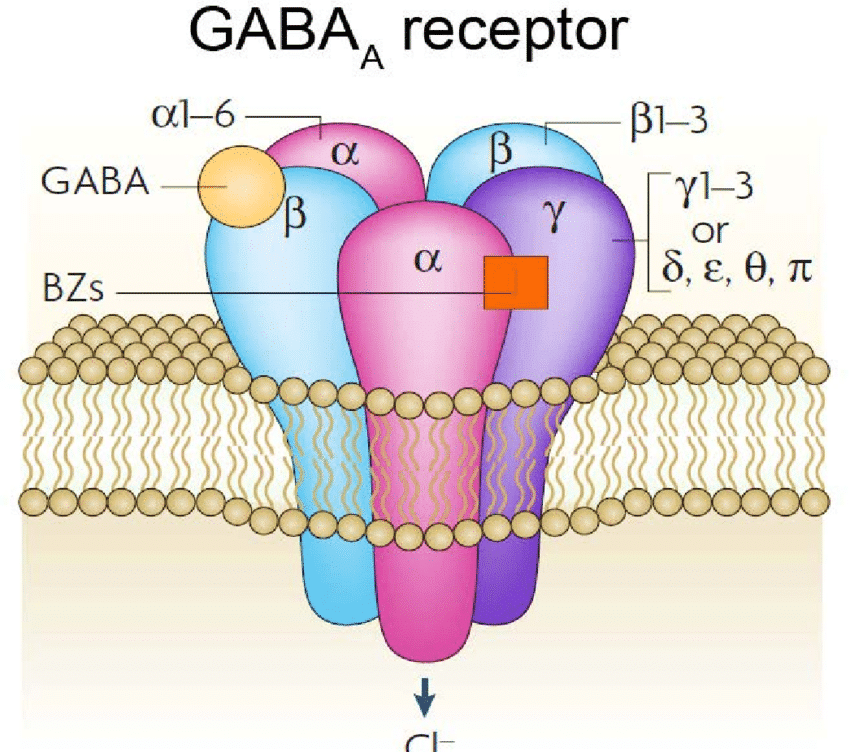
GABA Receptors
Ketamine’s effect on GABA: Potentiation (increased effect).
GABA (γ‐Amino‐butyrique acid) is the brain’s primary inhibiting neurotransmitter. It acts like the brake pedal for the brain — slowing everything down when it gets moving too fast.
Like many other drugs, ketamine has been shown to potentiate the effects of GABA [6]. It doesn’t stimulate these receptors; rather, it allows pre-existing GABA to exert a stronger force than it would on its own.
Many other drugs share this effect — such as alcohol, GHB (gamma-hydroxubutyric acid), phenibut, barbiturates, and benzodiazepines.
GABA potentiation results in a sense of calmness and relaxation in lower doses and sedation, intoxication, and amnesia in higher doses.
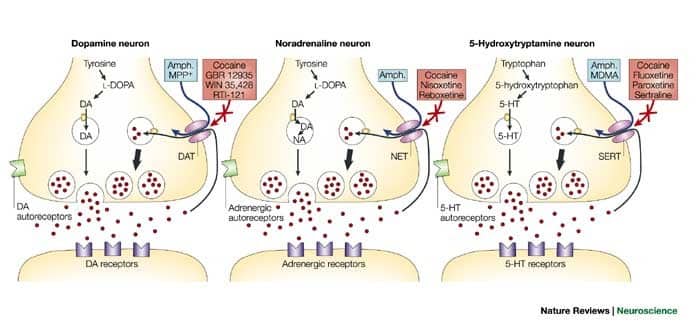
Monoaminergic Receptors (Dopamine, Serotonin, & Norepinephrine)
Ketamine’s effect on monoamines: Increases noradrenaline, alters serotonin and dopamine.
The monoamines are a group of neurotransmitters, including dopamine, serotonin, and norepinephrine.
Ketamine generally increases the activity of monoamine neurotransmitters — but it isn’t quite this simple. The trickle-down effects from other top-level interactions with ketamine can have a dramatically different impact on certain types of neurotransmitters in certain regions of the brain.
For example, ketamine stimulates the noradrenergic neurons in the locus coeruleus and inhibits its reuptake [7]. Dysfunctions in this region of the brain have been linked with altered fear responses, depression, and anxiety disorders [8].
Ketamine has also been shown to inhibit the reuptake of serotonin and dopamine [9]. As less monoamines are reabsorbed to be recycled, they accumulate in the synapses. This results in a reduced threshold for these neurotransmitters to exert their effect (in essence, it makes their effects stronger).
Ketamine’s impact on norepinephrine, dopamine, and serotonin are partially implicated in the hypnotic, psychedelic, and analgesic effects of the drug.
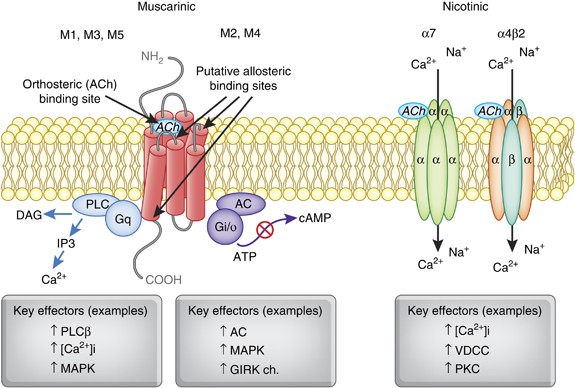
Cholinergic Receptors
The cholinergic system is a critical component of the nervous system and plays a variety of important roles in the body and brain.
Cholinergic neurons located throughout the body release a neurotransmitter called acetylcholine — which is responsible for various functions depending on the organ.
Outside the brain, the cholinergic system regulates functions like muscle contraction, heart rate, and glandular secretion.
Inside the brain, acetylcholine is involved in the process of learning, memory, attention, arousal, and many other aspects of higher brain function and behavior. Several well-known psychoactive drugs work by blocking this system — such as the tropane alkaloids from plants like datura, brugemansia, or mandrake.
There are two main types of cholinergic receptors that ketamine interacts with: the nicotinic receptors and the muscarinic receptors. These effects are significantly milder than compounds like tropane alkaloids but may play a role in ketamine’s overall effects nonetheless.
Studies have shown ketamine directly inhibits both the muscarinic and nicotinic receptors [10,11,12]. These target receptors, in part, allow ketamine to alter our ability to form new memories, affect heart rate and blood pressure, and alter our conscious perception of reality.
Some researchers believe the NMDA inhibitory effects of ketamine could also be a cause for acetylcholine blockage in the striatum, further contributing to this effect [13].
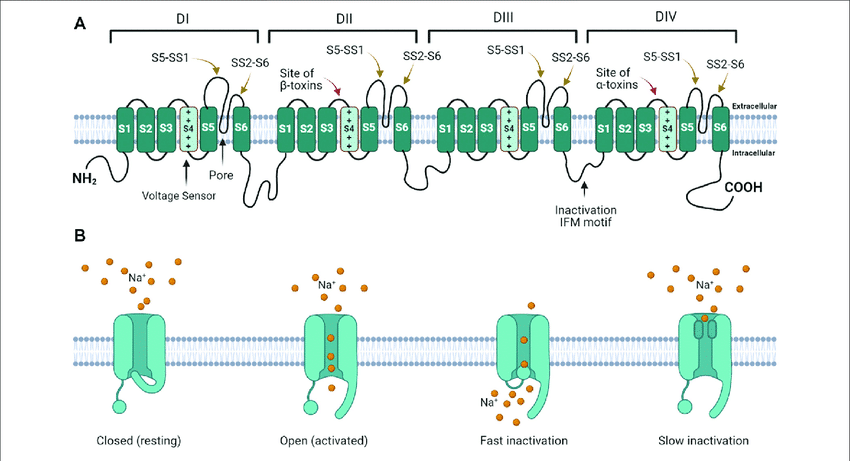
Sodium Channels
The sodium channels are special proteins located in our cells that are intimately involved in the movement of electrical signals.
Ketamine has been shown to block the sodium ion channels located in the brain [14], which slows down and inhibits electrical activity. In high enough doses, this effect is considered a key element of ketamine’s anesthetic properties.
Ketamine has also been shown to inhibit neuronal potassium channels [15], which further contributes to its ability to modulate neuronal excitability and induce a state of anesthesia.
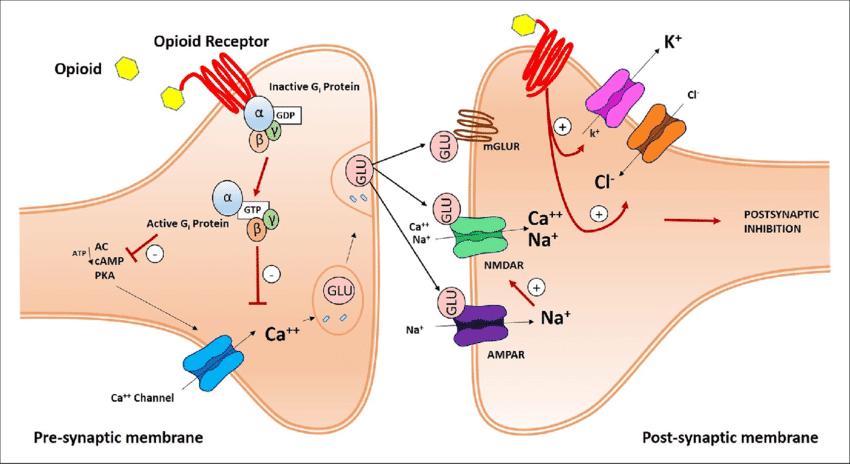
Opioid Receptors
Ketamine has been shown to interact with all three opioid receptor subtypes — mu-opioid, delta-opioid, and kappa-opioid receptors [16].
The opioid receptors are located in the brain and spinal cord and are responsible for regulating pain signals traveling toward the brain. Activation results in a dulled pain response. This is the same mechanism of action used by drugs like morphine and oxycodone that are used to block pain.
The opioid receptors are also involved in the reward pathway — which releases a dose of dopamine whenever we do something pleasurable. This is what gives opiate drugs (and ketamine, to a lesser extent) their euphoric qualities.
While ketamine has been found to target opioid receptors, this mechanism is not believed to play a key role in ketamine analgesic or antidepressant effects and is not reversed by naloxone. Its binding affinity overall for these receptors is very low.
Some of the drugs’ psychedelic and dissociative qualities are believed to stem from their ability to bind with the kappa-opioid receptors [16]. This is considered the mechanism of action for other psychedelic drugs, such as salvinorin A from the plant Salvia divinorum.
Interestingly, the S-enantiomer of ketamine was found to have a 2–3 times higher binding affinity for the opioid receptors than the R-enantiomer.
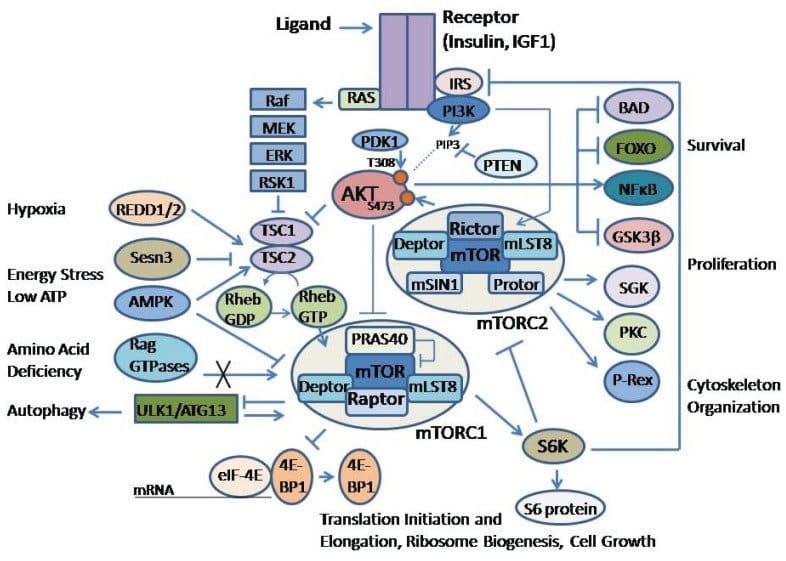
mTOR
mTOR (mammalian target of rapamycin) is a critical kinase enzyme involved in regulating cell growth and proliferation.
One study from Japan reported that ketamine “rapidly activates the mammalian target of mTOR, leading to an increase in synaptic signaling proteins (called spines) that can then go on to form new connections with other neurons [17].
In simple terms, ketamine appears to use the mTOR pathway to make neurons stickier so they can form new connections with other neurons. This neuroplastic effect is believed to play a key role in ketamine’s overall antidepressant, nootropic, and neuroprotective effects.
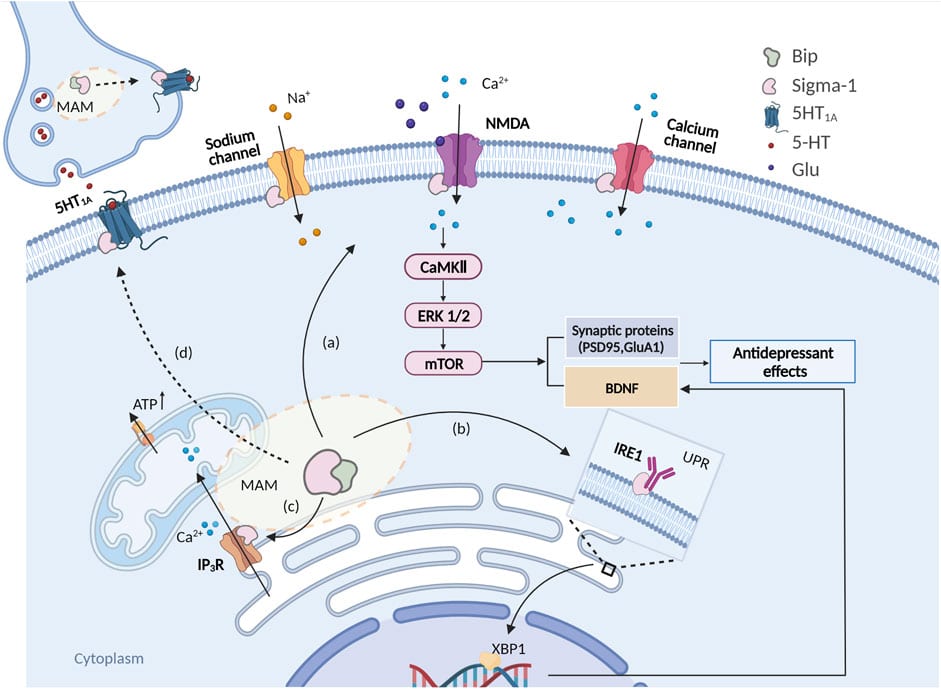
Sigma-1 & Sigma-2
The sigma receptors are a class of proteins found in the brain that are involved in a wide range of biological processes. Some research suggests they could even be implicated in the psychoactivity of certain drugs, including ketamine.
Sigma receptors have also been implicated in the progression of depression [21], and some research points to ketamine’s involvement with the Sigma-1 subtype, specifically, as an important mechanism for the drug’s antidepressant action [20].
Initially, researchers thought the sigma receptors were a new type of opioid receptor, but newer research has proven that these receptors are, in fact, distinct from the opiate system.
Mechanism of Action For Specific Ketamine Actions
Ketamine does many things in the human body. As we’ve outlined above, ketamine targets many different types of receptors to produce its combined effect.
Here, we’ll break down what mechanism is primarily involved with ketamine’s specific effects.
Antidepressant Mechanisms of Ketamine
Ketamine has been shown to produce impressive antidepressant action across numerous studies dating back to the early 2000s. Its effects are rapid but short-lived (lasting up to 2 weeks before fading away).
While researchers are still scrambling to understand the full extent of ketamine’s powerful antidepressant effects and why it works so well for some but not for others — there are a few good theories for what’s causing this effect:
The main mechanism is ketamine’s ability to enhance neuroplasticity. This pathway relies on ketamine’s impact on mTOR and AMPA receptors.
Dysfunctions in the mTOR pathway and other difficulties forming new synaptic connections in the prefrontal cortex and hippocampus have been found to play a key role in the development of chronic, treatment-resistant depression [18]. Ketamine’s unique ability to rapidly upregulate mTOR activity is a groundbreaking new approach for depression therapy.
The opioid-like effects of ketamine are also thought to play a role in the short-term improvements in mood from ketamine. Stimulating the opioid receptors causes a release of dopamine, which feels euphoric. This effect usually only lasts a few hours, but when combined with the other neuroplastic and antidepressant mechanisms of ketamine, it could remain in place for several days before fading away.
Another mechanism is the dissociative state induced by ketamine itself — during which users often experience a state described as “euphoric nothingness.” During this state, the user is aware of themselves but has lost all form of attachment to their identity. While in this headspace, users can take an unbiased look at themselves for who they truly are and identify some of the unconscious influences that have been controlling the way they think and act.
In Jungian psychology, this is called the shadow.
Removing these unconscious elements that often drive depression and anxiety requires us to identify them first — followed by support from a skilled therapist to integrate these aspects into our conscious mind so they no longer influence us from the sidelines.
Analgesic Mechanisms of Ketamine
Ketamine is often used as a painkiller, even though it isn’t as effective as conventional opiate drugs. Some of ketamine’s mechanism of action relies on its opiate-like effects, but this is only a minor aspect compared to the drug’s other effects.
With that said, ketamine can be a powerful painkiller for people with specific types of pain conditions. Most notably, pain caused by the “wind-up phenomenon.”
This condition refers to a gradual increase in pain response to stimuli that are normally not painful or are only mildly painful. It’s believed to be involved with some chronic, often untreatable, pain conditions such as fibromyalgia, migraine headaches, and CRPS.
Ketamine’s ability to reduce or even prevent wind-up pain conditions from taking hold relies on its effects on the NMDA receptors.

Dissociative Mechanisms of Ketamine
Ketamine’s dissociative effects are primarily caused by its effects on the NMDA receptors.
Other NMDA inhibitors cause similar effects — even the ones that have nothing else in common with ketamine, such as DXM (dextromethorphan), xenon, and nitrous oxide.
Some theories suggest this disconnection results from interruptions in communication between different regions of the brain — particularly those involved in sensory perception and self-awareness.
Other mechanisms that play a role in the dissociative effect of ketamine are the kappa-opioid receptors, inhibition of sodium ion channels, and modulations in serotonin and dopamine systems.
Ketamine Mechanism of Action FAQs
Here are some of the common questions we get about ketamine — specifically about how it works.
1. Which form of ketamine is the most active?
There are two enantiomers of ketamine — S-ketamine and R-ketamine. The S enantiomer is roughly two times stronger than the R enantiomer.
An enantiomer refers to a mirror image of another form of the same molecule, but they cannot be superimposed on each other.
You can think of it like your left and right hands. They are identical but are mirror images of each other. You cannot superimpose them unless you flip one hand upside down.
2. What is the half-life of ketamine?
The half-life of ketamine ranges from 2 to 3 hours. Most sources list the average as 2.5 hours.
Related: How Does Ketamine Remain in the Body?
3. Does ketamine interact with serotonin?
Yes, ketamine alters serotonin balance as a secondary byproduct of its primary mechanism of action on the NMDA receptors. These receptors are involved with the regulation and signaling pathways of serotonin.
4. Does ketamine interact with dopamine?
Yes, ketamine interacts with dopamine indirectly by stimulating the opiate receptors. The opiate system is responsible for regulating pain transmissions as they travel up the spinal cord and into the brain — but they also regulate the reward cycle in the brain. This system relies heavily on dopamine to function.
Basically, when we do something that benefits the body — such as eating or having sex — the reward system kicks in to give us a dose of dopamine. This dopamine then causes the release of other neurotransmitters that give us a sudden feeling of euphoria before fading away. We unconsciously seek out activities that provide more of this euphoric effect — thus reinforcing the habit.
5. How is ketamine administered?
Ketamine can be administered in several different ways — orally by swallowing a capsule or tablet, intranasally via a nasal spray or by snorting the raw powder, sublingually using lozenges or troches that absorb through capillaries under the tongue, through injection into the muscles (IM) or directly into the veins (IV), or rectally through the use of an enema.
Subscribe to Tripsitter: Newsletter & Podcast
Unlock Your Mind: Subscribe for Expert Insights on Psychedelics 🍄🌵
References
- Sleigh, J., Harvey, M., Voss, L., & Denny, B. (2014). Ketamine–More mechanisms of action than just NMDA blockade. Trends in anaesthesia and critical care, 4(2-3), 76-81. https://doi.org/10.1016/j.tacc.2014.03.002
- Herrero, J. F., Laird, J. M., & Lopez-Garcia, J. A. (2000). Wind-up of spinal cord neurones and pain sensation: much ado about something?. Progress in neurobiology, 61(2), 169-203. https://doi.org/10.1016/S0301-0082(99)00051-9
- Yang, C., Kobayashi, S., Nakao, K., Dong, C., Han, M., Qu, Y., … & Hashimoto, K. (2018). AMPA receptor activation–independent antidepressant actions of ketamine metabolite (S)-norketamine. Biological psychiatry, 84(8), 591-600. https://doi.org/10.1016/j.biopsych.2018.05.007
- Aleksandrova, L. R., Wang, Y. T., & Phillips, A. G. (2017). Hydroxynorketamine: implications for the NMDA receptor hypothesis of ketamine’s antidepressant action. Chronic Stress, 1, 2470547017743511. https://doi.org/10.1177/2470547017743511
- Suzuki, A., Hara, H., & Kimura, H. (2023). Role of the AMPA receptor in antidepressant effects of ketamine and potential of AMPA receptor potentiators as a novel antidepressant. Neuropharmacology, 222, 109308. https://doi.org/10.1016/j.neuropharm.2022.109308
- Pham, T. H., & Gardier, A. M. (2019). Fast-acting antidepressant activity of ketamine: highlights on brain serotonin, glutamate, and GABA neurotransmission in preclinical studies. Pharmacology & therapeutics, 199, 58-90. https://doi.org/10.1016/j.pharmthera.2019.02.017
- Levanen, J., Makela, M. L., & Scheinin, H. (1995). Dexmedetomidine premedication attenuates ketamine-induced cardiostimulatory effects and postanesthetic delirium. The Journal of the American Society of Anesthesiologists, 82(5), 1117-1125. https://doi.org/10.1097/00000542-199505000-00005
- Weiss, J. M., Stout, J. C., Aaron, M. F., Quan, N., Owens, M. J., Butler, P. D., & Nemeroff, C. B. (1994). Depression and anxiety: role of the locus coeruleus and corticotropin-releasing factor. Brain Research Bulletin, 35(5-6), 561-572. https://doi.org/10.1016/0361-9230(94)90170-8
- Martin, L. L., Bouchal, R. L., & Smith, D. J. (1982). Ketamine inhibits serotonin uptake in vivo. Neuropharmacology, 21(2), 113-118. https://doi.org/10.1016/0028-3908(82)90149-6
- Toro-Matos, A., Rendon-Platas, A. M., Avila-Valdez, E., & Villarreal-Guzman, R. A. (1980). Physostigmine antagonizes ketamine. Anesthesia & Analgesia, 59(10), 764-767.
- Furuya, R., Oka, K., Watanabe, I., Kamiya, Y., Itoh, H., & Andoh, T. (1999). The effects of ketamine and propofol on neuronal nicotinic acetylcholine receptors and P2x purinoceptors in PC12 cells. Anesthesia & Analgesia, 88(1), 174-180. https://doi.org/10.1213/00000539-199901000-00033
- Durieux, M. E. (1995). Inhibition by ketamine of muscarinic acetylcholine receptor function. Anesthesia & Analgesia, 81(1), 57-62.
- Kohrs, R., & Durieux, M. E. (1998). Ketamine: teaching an old drug new tricks. Anesthesia & Analgesia, 87(5), 1186-1193. https://doi.org/10.1213/00000539-199811000-00039
- Frenkel, C., & Urban, B. W. (1992). Molecular actions of racemic ketamine on human CNS sodium channels. British journal of anaesthesia, 69(3), 292-297. https://doi.org/10.1093/bja/69.3.292
- Friederich, P., & Urban, B. W. (1999). Interaction of intravenous anesthetics with human neuronal potassium currents in relation to clinical concentrations. The Journal of the American Society of Anesthesiologists, 91(6), 1853-1853. https://doi.org/10.1097/00000542-199912000-000402
- Hustveit, O., Maurset, A., & Øye, I. (1995). Interaction of the chiral forms of ketamine with opioid, phencyclidine, σ, and muscarinic receptors. Pharmacology & toxicology, 77(6), 355-359. https://doi.org/10.1111/j.1600-0773.1995.tb01041.x
- Hashimoto, K. (2011). Role of the mTOR signaling pathway in the rapid antidepressant action of ketamine. Expert Review of Neurotherapeutics, 11(1), 33-36. https://doi.org/10.1586/ern.10.176
- Jernigan, C. S., Goswami, D. B., Austin, M. C., Iyo, A. H., Chandran, A., Stockmeier, C. A., & Karolewicz, B. (2011). The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 35(7), 1774-1779. https://doi.org/10.1016/j.pnpbp.2011.05.010
- Mion, G., & Villevieille, T. (2013). Ketamine pharmacology: an update (pharmacodynamics and molecular aspects, recent findings). CNS neuroscience & therapeutics, 19(6), 370-380. https://doi.org/10.1111/cns.12099
- Robson, M. J., Elliott, M., Seminerio, M. J., & Matsumoto, R. R. (2012). Evaluation of sigma (σ) receptors in the antidepressant-like effects of ketamine in vitro and in vivo. European Neuropsychopharmacology, 22(4), 308-317.
- Nguyen, L., Robson, M. J., Healy, J. R., Scandinaro, A. L., & Matsumoto, R. R. (2014). Involvement of sigma-1 receptors in the antidepressant-like effects of dextromethorphan. PloS one, 9(2), e89985. https://doiorg/10.1371/journal.pone.0089985